Genomic Characterization of the Historical Smallpox Vaccine Strain Wyeth Isolated from a 1971 Seed Vial
- PMID: 36680123
- PMCID: PMC9864299
- DOI: 10.3390/v15010083
Genomic Characterization of the Historical Smallpox Vaccine Strain Wyeth Isolated from a 1971 Seed Vial
Abstract
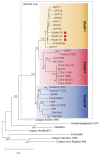
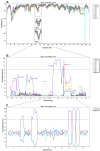
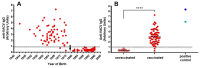
The Wyeth strain of vaccinia virus (VACV) produced by Wyeth Pharmaceuticals was supposedly used to manufacture the old freeze-dried American smallpox vaccine, Dryvax, until its discontinuation in 2008. Although the genomic sequences of numerous Dryvax clones have been reported, data on VACV-Wyeth genomes are still lacking. Genomic analysis of old VACV strains is relevant to understand the evolutionary relationships of smallpox vaccines, particularly with the recent resumption of smallpox vaccination in certain population groups as an attempt to control the worldwide monkeypox outbreak. Here we analyzed the complete genome sequences of three VACV-Wyeth clonal isolates obtained from a single seed vial donated to the Brazilian eradication program in the 1970s. Wyeth clones show >99.3% similarity to each other and >95.3% similarity with Dryvax clones, mapping together in clade I of the vaccinia group. Although the patterns of SNPs and INDELs comparing Dryvax and Wyeth clones are overall uniform, important differences were detected particularly at the ends of the genome. In addition, we detected recombinant events of clone Wyeth A111 and the Dryvax clone Acam2000, suggesting that other regions of the genomes may have similar patchy patterns of recombination. A small-scale serological survey using VACV-Wyeth as antigen in ELISA assays revealed that 63 of the 65 individuals born before the end of smallpox vaccination in Brazil still have anti-VACV IgG antibodies, demonstrating the usefulness of the VACV-Wyeth strain in future extended serological studies of the Brazilian population.
Keywords: monkeypox virus; poxvirus; smallpox vaccine; vaccinia virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Genomic Analysis, Phenotype, and Virulence of the Historical Brazilian Smallpox Vaccine Strain IOC: Implications for the Origins and Evolutionary Relationships of Vaccinia Virus.J Virol. 2015 Dec;89(23):11909-25. doi: 10.1128/JVI.01833-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378174 Free PMC article.
-
Genomic sequence and analysis of a vaccinia virus isolate from a patient with a smallpox vaccine-related complication.Virol J. 2006 Oct 25;3:88. doi: 10.1186/1743-422X-3-88. Virol J. 2006. PMID: 17062162 Free PMC article.
-
Genomic differences of Vaccinia virus clones from Dryvax smallpox vaccine: the Dryvax-like ACAM2000 and the mouse neurovirulent Clone-3.Vaccine. 2007 Dec 17;25(52):8807-32. doi: 10.1016/j.vaccine.2007.10.040. Epub 2007 Nov 8. Vaccine. 2007. PMID: 18037545
-
ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile.Drug Des Devel Ther. 2010 May 25;4:71-9. doi: 10.2147/dddt.s3687. Drug Des Devel Ther. 2010. PMID: 20531961 Free PMC article. Review.
-
ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)--a second-generation smallpox vaccine for biological defense.Int J Infect Dis. 2004 Oct;8 Suppl 2:S31-44. doi: 10.1016/j.ijid.2004.09.002. Int J Infect Dis. 2004. PMID: 15491873 Free PMC article. Review.
Cited by
-
Progress and prospects on vaccine development against monkeypox infection.Microb Pathog. 2023 Jul;180:106156. doi: 10.1016/j.micpath.2023.106156. Epub 2023 May 16. Microb Pathog. 2023. PMID: 37201635 Free PMC article. Review.
References
-
- Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D. Smallpox and Its Eradication. World Health Organization; Geneva, Switzerland: 1988. p. 1460.
-
- Rutty C. Canadian vaccine research, production and international regulation: Connaught Laboratories and Smallpox Vaccines,1962–1980. In: Kroker K., Kealan J., Mazumdar P.M., editors. Crafting Immunity: Working Histories of Clinical Immunology. Ashgate Publishing, Ltd.; Burlington, NJ, USA: 2008. pp. 280–289.
-
- FDA. [(accessed on 28 October 2022)]; Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/smallpox.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

