Clinical Landscape and Rate of Exposure to Ilheus Virus: Insights from Systematic Review and Meta-Analysis
- PMID: 36680131
- PMCID: PMC9861323
- DOI: 10.3390/v15010092
Clinical Landscape and Rate of Exposure to Ilheus Virus: Insights from Systematic Review and Meta-Analysis
Abstract
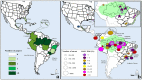
Ilheus fever is a mosquito-borne, poorly known tropical disease. We aimed to report the pooled rate of exposure to the Ilheus virus (ILHV) and clinical outcomes of infection to determine the epidemiological patterns of ILHV. We conducted a meta-analysis of 37 studies (n = 17,722 individuals) from Latin America. The common clinical characteristics of ILHV infection were fever (82.3%), headache (52.9%), and myalgia (52.9%). Encephalitis complicated the course of the infection in 29.4% cases. Monotypic serological reactions detected a pooled rate of exposure of 2% to ILHV (95% CI: 1-2). Studies were mainly conducted in Brazil, with a pooled proportion of ILHV positivity of 8% (95% CI: 3-14). Males (12%) had higher rates of seropositivity than females (7%) and had high chances of ILHV infection (OR: 1.7, 95% CI: 1.2-2.5). Seropositivity increased with age, from 2% (95% CI: 2-3) among people aged 0-14 years to 8% (95% CI: 6-10) among people aged 15-64 years. Our analysis indicated a low and relatively constant burden of ILHV in Latin America. More research is needed to evaluate and innovate serological assays for ILHV to better estimate the burden and dynamics of epidemiological changes in ILHV infection in different regions.
Keywords: Ilheus virus; arbovirus; meta-analysis; neglected disease; sero-epidemiology.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
In Vitro System for Studying Ilhéus Virus, a Neglected Arbovirus: Ultrastructural Characterization of Cytopathology, Morphology, and Morphogenesis.Viruses. 2025 Feb 26;17(3):320. doi: 10.3390/v17030320. Viruses. 2025. PMID: 40143249 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2022 May 16;5(5):CD013639. doi: 10.1002/14651858.CD013639.pub5. Cochrane Database Syst Rev. 2022. PMID: 35575286 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
Cited by
-
(Re)Emerging Arboviruses of Public Health Significance in the Brazilian Amazon.Microorganisms. 2025 Mar 12;13(3):650. doi: 10.3390/microorganisms13030650. Microorganisms. 2025. PMID: 40142542 Free PMC article. Review.
-
Zoonotic Orthoflaviviruses Related to Birds: A Literature Review.Microorganisms. 2025 Jul 6;13(7):1590. doi: 10.3390/microorganisms13071590. Microorganisms. 2025. PMID: 40732099 Free PMC article. Review.
-
Production of a chimeric flavivirus that contains the major structural glycoprotein genes of T'Ho virus in the genetic background of Zika virus.Virol J. 2023 Sep 1;20(1):197. doi: 10.1186/s12985-023-02172-2. Virol J. 2023. PMID: 37658438 Free PMC article.
-
Potential of Ilhéus virus to emerge.Heliyon. 2024 Mar 16;10(6):e27934. doi: 10.1016/j.heliyon.2024.e27934. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38545168 Free PMC article.
-
In Vitro System for Studying Ilhéus Virus, a Neglected Arbovirus: Ultrastructural Characterization of Cytopathology, Morphology, and Morphogenesis.Viruses. 2025 Feb 26;17(3):320. doi: 10.3390/v17030320. Viruses. 2025. PMID: 40143249 Free PMC article.
References
-
- Laemmert J.R., Hugo W., Hughes, Thomas P. The virus of Ilheus encephalitis: Isolation, serological specificity and trans-mission. J. Immunol. 1947;55:61–67. - PubMed
-
- Saivish M.V., Menezes G.L., Costa V.G.D., Silva G.C.D.D., Marques R.E., Nogueira M.L., Silva R.A.D. Predict-ing Antigenic Peptides from Rocio Virus NS1 Protein for Immunodiagnostic Testing Using Immunoinformatics and Molecular Dynamics Simulation. Int. J. Mol. Sci. 2022;23:7681. doi: 10.3390/ijms23147681. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

