Mouse Adapted SARS-CoV-2 (MA10) Viral Infection Induces Neuroinflammation in Standard Laboratory Mice
- PMID: 36680154
- PMCID: PMC9863644
- DOI: 10.3390/v15010114
Mouse Adapted SARS-CoV-2 (MA10) Viral Infection Induces Neuroinflammation in Standard Laboratory Mice
Abstract
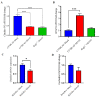
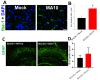
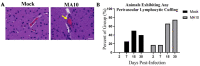
Increasing evidence suggests that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection impacts neurological function both acutely and chronically, even in the absence of pronounced respiratory distress. Developing clinically relevant laboratory mouse models of the neuropathogenesis of SARS-CoV-2 infection is an important step toward elucidating the underlying mechanisms of SARS-CoV-2-induced neurological dysfunction. Although various transgenic models and viral delivery methods have been used to study the infection potential of SARS-CoV-2 in mice, the use of commonly available laboratory mice would facilitate the study of SARS-CoV-2 neuropathology. Herein we show neuroinflammatory profiles of immunologically intact mice, C57BL/6J and BALB/c, as well as immunodeficient (Rag2-/-) mice, to a mouse-adapted strain of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2 (MA10)). Our findings indicate that brain IL-6 levels are significantly higher in BALB/c male mice infected with SARS-CoV-2 MA10. Additionally, blood-brain barrier integrity, as measured by the vascular tight junction protein claudin-5, was reduced by SARS-CoV-2 MA10 infection in all three strains. Brain glial fibrillary acidic protein (GFAP) mRNA was also elevated in male C57BL/6J infected mice compared with the mock group. Lastly, immune-vascular effects of SARS-CoV-2 (MA10), as measured by H&E scores, demonstrate an increase in perivascular lymphocyte cuffing (PLC) at 30 days post-infection among infected female BALB/c mice with a significant increase in PLC over time only in SARS-CoV-2 MA10) infected mice. Our study is the first to demonstrate that SARS-CoV-2 (MA10) infection induces neuroinflammation in laboratory mice and could be used as a novel model to study SARS-CoV-2-mediated cerebrovascular pathology.
Keywords: COVID-19; SARS-CoV-2; animal models; brain; mouse-adaptation; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Amruta N., Chastain W.H., Paz M., Solch R.J., Murray-Brown I.C., Befeler J.B., Gressett T.E., Longo M.T., Engler-Chiurazzi E.B., Bix G. SARS-CoV-2 mediated neuroinflammation and the impact of COVID-19 in neurological disorders. Cytokine Growth Factor Rev. 2021;58:1–15. doi: 10.1016/j.cytogfr.2021.02.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

