Monoclonal Antibodies Specific for SARS-CoV-2 Spike Protein Suitable for Multiple Applications for Current Variants of Concern
- PMID: 36680179
- PMCID: PMC9863740
- DOI: 10.3390/v15010139
Monoclonal Antibodies Specific for SARS-CoV-2 Spike Protein Suitable for Multiple Applications for Current Variants of Concern
Abstract
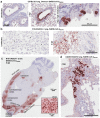
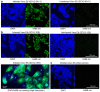
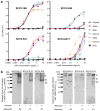
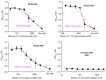
The global coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spawned an ongoing demand for new research reagents and interventions. Herein we describe a panel of monoclonal antibodies raised against SARS-CoV-2. One antibody showed excellent utility for immunohistochemistry, clearly staining infected cells in formalin-fixed and paraffin embedded lungs and brains of mice infected with the original and the omicron variants of SARS-CoV-2. We demonstrate the reactivity to multiple variants of concern using ELISAs and describe the use of the antibodies in indirect immunofluorescence assays, Western blots, and rapid antigen tests. Finally, we illustrate the ability of two antibodies to reduce significantly viral tissue titers in K18-hACE2 transgenic mice infected with the original and an omicron isolate of SARS-CoV-2.
Keywords: SARS-CoV-2; Western blotting; immunofluorescence; immunohistochemistry; immunotherapy; lateral flow assays; monoclonal antibody.
Conflict of interest statement
One or more of the mAbs may be sold under commercial agreement(s) with some revenue returning to the authors.
Figures

References
-
- Best Rocha A., Stroberg E., Barton L.M., Duval E.J., Mukhopadhyay S., Yarid N., Caza T., Wilson J.D., Kenan D.J., Kuperman M., et al. Detection of SARS-CoV-2 in formalin-fixed paraffin-embedded tissue sections using commercially available reagents. Lab. Investig. 2020;100:1485–1489. doi: 10.1038/s41374-020-0464-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

