Seroprevalence of Natural and Acquired Immunity against the SARS-CoV-2 Virus in a Population Cohort from Two Chilean Cities, 2020-2022
- PMID: 36680241
- PMCID: PMC9861850
- DOI: 10.3390/v15010201
Seroprevalence of Natural and Acquired Immunity against the SARS-CoV-2 Virus in a Population Cohort from Two Chilean Cities, 2020-2022
Abstract
Background: Chile has achieved the highest coverage for vaccines against the SARS-CoV-2 virus worldwide.
Objective: To assess the progression of immunity (natural and acquired by vaccine) in a cohort from two Chilean cities.
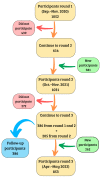
Methods: Individuals (n = 386) who participated in three phases of population-based serial prevalence studies were included (2020-2021 and 2022). Presence of SARS-CoV-2 antibodies was measured in serum. Data including time of vaccination and type of vaccine received were analysed with descriptive statistics.
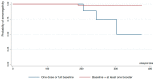
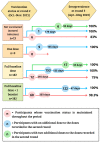
Results: Seroprevalence was 3.6% in the first round and increased to 96.9% in the second and 98.7% in the third. In the third round, 75% of individuals who had received the basal full scheme were seropositive at 180 days or more since their last dose; 98% of individuals who received one booster dose were seropositive at 180 days or more, and 100% participants who received two boosters were seropositive, regardless of time since their last dose. Participants receiving mRNA vaccines had higher seroprevalence rates over time.
Conclusions: The high vaccination coverage in Chile enabled the population to maintain high levels of antibodies. Vaccination boosters are essential to maintain immunity over time, which also depends on the type of vaccine administered.
Keywords: COVID-19; Chile; antibodies; immunity; seroepidemiology; seroprevalence; vaccines.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
COVID-19 lateral flow IgG seropositivity and serum neutralising antibody responses after primary and booster vaccinations in Chile: a cross-sectional study.Lancet Microbe. 2023 Mar;4(3):e149-e158. doi: 10.1016/S2666-5247(22)00290-7. Epub 2023 Jan 27. Lancet Microbe. 2023. PMID: 36716754 Free PMC article.
-
Population-based seroprevalence survey: post-pandemic COVID-19 vaccination, related factors, and geographic distribution of vaccine acceptability in Chile.BMC Public Health. 2025 Mar 28;25(1):1176. doi: 10.1186/s12889-025-22314-1. BMC Public Health. 2025. PMID: 40155965 Free PMC article.
-
Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study.Lancet Infect Dis. 2022 Jan;22(1):56-63. doi: 10.1016/S1473-3099(21)00479-5. Epub 2021 Sep 9. Lancet Infect Dis. 2022. PMID: 34509185 Free PMC article.
-
Humoral immunity against SARS-CoV-2 evoked by heterologous vaccination groups using the CoronaVac (Sinovac) and BNT162b2 (Pfizer/BioNTech) vaccines in Chile.Front Public Health. 2023 Aug 24;11:1229045. doi: 10.3389/fpubh.2023.1229045. eCollection 2023. Front Public Health. 2023. PMID: 37693706 Free PMC article.
-
Seroprevalence of SARS-CoV-2 and humoral immune responses to COVID-19 mRNA vaccines among people who use drugs - in the light of tailored mitigating strategies.Harm Reduct J. 2024 Jun 19;21(1):120. doi: 10.1186/s12954-024-01023-9. Harm Reduct J. 2024. PMID: 38890611 Free PMC article.
Cited by
-
Overcoming Health Inequities: Spatial Analysis of Seroprevalence and Vaccination Against COVID-19 in Chile.Health Equity. 2024 Aug 26;8(1):558-567. doi: 10.1089/heq.2023.0204. eCollection 2024. Health Equity. 2024. PMID: 40125367 Free PMC article.
-
Seroprevalence of SARS-CoV-2 anti-nucleocapsid total Ig, anti-RBD IgG antibodies, and infection in Thailand: a cross-sectional survey from October 2022 to January 2023.Sci Rep. 2023 Sep 20;13(1):15595. doi: 10.1038/s41598-023-42754-2. Sci Rep. 2023. PMID: 37730917 Free PMC article.
-
Increased vaccine sensitivity of an emerging SARS-CoV-2 variant.Nat Commun. 2023 Jun 29;14(1):3854. doi: 10.1038/s41467-023-39567-2. Nat Commun. 2023. PMID: 37386005 Free PMC article.
-
Factors influencing neutralizing antibody response to the original SARS-CoV-2 virus and the Omicron variant in a high vaccination coverage country, a population-based study.Vaccine X. 2023 Aug 26;15:100372. doi: 10.1016/j.jvacx.2023.100372. eCollection 2023 Dec. Vaccine X. 2023. PMID: 37693843 Free PMC article.
-
The Second Round of a Population-Based Seroprevalence Study of Anti-SARS-CoV-2 Antibodies and COVID-19 Vaccination Assessment in the Republika Srpska, Bosnia and Herzegovina.Influenza Other Respir Viruses. 2025 Jan;19(1):e70053. doi: 10.1111/irv.70053. Influenza Other Respir Viruses. 2025. PMID: 39823154 Free PMC article.
References
-
- World Health Organization WHO Coronavirus Dashboard. WHO Web Page COVID-19 Data. [(accessed on 19 October 2022)]. Available online: https://covid19.who.int/
-
- Metcalf C.J.E., Farrar J., Cutts P.F.T., Basta N., Graham A., Lessler J., Ferguson N., Burke and D., Grenfell B. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet. 2016;388:728–730. doi: 10.1016/S0140-6736(16)30164-7. - DOI - PMC - PubMed
-
- Vial P., González C., Icaza G., Ramírez-Santana M., Quezada-Gaete R., Núñez-Franz L., Apablaza M., Vial C., Rubilar P., Correa J., et al. Seroprevalence, spatial distribution, and social determinants of SARS-CoV-2 in three urban centers of Chile. BMC Infect. Dis. 2022;22:99. doi: 10.1186/s12879-022-07045-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

