Development of HIV-Resistant CAR T Cells by CRISPR/Cas-Mediated CAR Integration into the CCR5 Locus
- PMID: 36680242
- PMCID: PMC9862650
- DOI: 10.3390/v15010202
Development of HIV-Resistant CAR T Cells by CRISPR/Cas-Mediated CAR Integration into the CCR5 Locus
Abstract
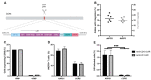
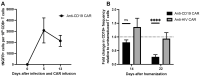
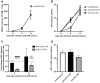
Adoptive immunotherapy using chimeric antigen receptor (CAR) T cells has been highly successful in treating B cell malignancies and holds great potential as a curative strategy for HIV infection. Recent advances in the use of anti-HIV broadly neutralizing antibodies (bNAbs) have provided vital information for optimal antigen targeting of CAR T cells. However, CD4+ CAR T cells are susceptible to HIV infection, limiting their therapeutic potential. In the current study, we engineered HIV-resistant CAR T cells using CRISPR/Cas9-mediated integration of a CAR cassette into the CCR5 locus. We used a single chain variable fragment (scFv) of the clinically potent bNAb 10-1074 as the antigen-targeting domain in our anti-HIV CAR T cells. Our anti-HIV CAR T cells showed specific lysis of HIV-infected cells in vitro. In a PBMC humanized mouse model of HIV infection, the anti-HIV CAR T cells expanded and transiently limited HIV infection. In conclusion, this study provides proof-of-concept for developing HIV-resistant CAR T cells using CRISPR/Cas9 targeted integration.
Keywords: CAR T cells; CRISPR/Cas9; HIV; humanized mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mitsuyasu R.T., Anton P.A., Deeks S.G., Scadden D.T., Connick E., Downs M.T., Bakker A., Roberts M.R., June C.H., Jalali S., et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4ζ gene-modified autologous CD4+ and CD8+ T cells in human immunodeficiency virus–infected subjects. Blood. 2000;96:785–793. doi: 10.1182/blood.V96.3.785.015k10_785_793. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

