Exploring the Expression and Function of cTyro3, a Candidate Zika Virus Receptor, in the Embryonic Chicken Brain and Inner Ear
- PMID: 36680287
- PMCID: PMC9867072
- DOI: 10.3390/v15010247
Exploring the Expression and Function of cTyro3, a Candidate Zika Virus Receptor, in the Embryonic Chicken Brain and Inner Ear
Abstract
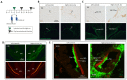
The transmembrane protein Axl was proposed as an entry receptor for Zika virus (ZIKV) infection in vitro, but conflicting results from in vivo studies have made it difficult to establish Axl as a physiologically relevant ZIKV receptor. Both the functional redundancy of receptors and the experimental model used can lead to variable results. Therefore, it can be informative to explore alternative animal models to analyze ZIKV receptor candidates as an aid in discovering antivirals. This study used chicken embryos to examine the role of chicken Tyro3 (cTyro3), the equivalent of human Axl. Results show that endogenous cTyro3 mRNA expression overlaps with previously described hot spots of ZIKV infectivity in the brain and inner ear. We asked if ectopic expression or knockdown of cTyro3 influenced ZIKV infection in embryos. Tol2 vectors or replication-competent avian retroviruses were used in ovo to introduce full-length or truncated (presumed dominant-negative) cTyro3, respectively, into the neural tube on embryonic day two (E2). ZIKV was delivered to the brain 24 h later. cTyro3 manipulations did not alter ZIKV infection or cell death in the E5/E6 brain. Moreover, delivery of truncated cTyro3 variants to the E3 otocyst had no effect on inner ear formation on E6 or E10.
Keywords: Axl; Tyro3; Zika; basilar papilla; neural tube; otocyst.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Axl Promotes Zika Virus Entry and Modulates the Antiviral State of Human Sertoli Cells.mBio. 2019 Jul 16;10(4):e01372-19. doi: 10.1128/mBio.01372-19. mBio. 2019. PMID: 31311882 Free PMC article.
-
A single mutation in the PrM gene of Zika virus determines AXL dependency for infection of human neural cells.J Virol. 2025 Apr 15;99(4):e0187324. doi: 10.1128/jvi.01873-24. Epub 2025 Mar 10. J Virol. 2025. PMID: 40062839 Free PMC article.
-
Axl is not an indispensable factor for Zika virus infection in mice.J Gen Virol. 2017 Aug;98(8):2061-2068. doi: 10.1099/jgv.0.000886. Epub 2017 Aug 8. J Gen Virol. 2017. PMID: 28786784 Free PMC article.
-
Zika Virus-Induced Microcephaly and Its Possible Molecular Mechanism.Intervirology. 2016;59(3):152-158. doi: 10.1159/000452950. Epub 2017 Jan 13. Intervirology. 2016. PMID: 28081529 Review.
-
AXL, an Important Host Factor for DENV and ZIKV Replication.Front Cell Infect Microbiol. 2021 Mar 22;11:575346. doi: 10.3389/fcimb.2021.575346. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33954117 Free PMC article. Review.
References
-
- Schuler-Faccini L., Ribeiro E.M., Feitosa I.M.L., Horovitz D.D., Cavalcanti D.P., Pessoa A., Doriqui M.J.R., Neri J.I., Neto J.M.D.P., Wanderley H.Y., et al. Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016;65:59–62. doi: 10.15585/mmwr.mm6503e2. - DOI - PubMed
-
- Brasil P., Pereira J.P., Jr., Moreira M.E., Nogueira R.M.R., Damasceno L., Wakimoto M., Rabello R.S., Valderramos S.G., Halai U.-A., Salles T.S., et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro-Preliminary report. N. Engl. J. Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. - DOI - PMC - PubMed
-
- Martines R.B., Bhatnagar J., Keating M.K., Silva-Flannery L., Muehlenbachs A., Gary J., Goldsmith C., Hale G., Ritter J., Rollin D., et al. Notes from the Field: Evidence of Zika Virus Infection in Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses—Brazil, 2015. MMWR. Morb. Mortal. Wkly. Rep. 2016;65:159–160. doi: 10.15585/mmwr.mm6506e1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

