Early and late preterm birth rates in participants adherent to randomly assigned high dose docosahexaenoic acid (DHA) supplementation in pregnancy
- PMID: 36680919
- PMCID: PMC10546372
- DOI: 10.1016/j.clnu.2023.01.009
Early and late preterm birth rates in participants adherent to randomly assigned high dose docosahexaenoic acid (DHA) supplementation in pregnancy
Abstract
Background: Intention-to-treat analyses do not address adherence. Per protocol analyses treat nonadherence as a protocol deviation and assess if the intervention is effective if followed.
Objective: To determine the rate of early preterm birth (EPTB, <34 weeks gestation) and preterm birth (PTB, <37 weeks gestation) in participants who adhered to a randomly assigned docosahexaenoic acid (DHA) dose of 1000 mg/day.
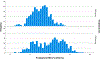
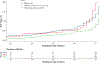
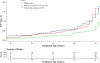
Study design: Eleven hundred women with a singleton pregnancy were enrolled before 20-weeks' gestation, provided a capsule with 200 mg/day DHA and randomly assigned to two additional capsules containing a placebo or 800 mg of DHA. In the Bayesian Adaptive Design, new randomization schedules were determined at prespecified intervals. In each randomization, the group with the most EPTB was assigned fewer participants than the other group. Adherence was defined a priori as a postpartum red blood cell phospholipid DHA (RBC-PL-DHA) ≥5.5%.and post hoc as ≥8.0% RBC-PL-DHA, the latter after examination of postpartum RBC-PL-DHA. Bayesian mixture models were fitted for gestational age and dichotomized for EPTB and PTB as a function of baseline RBC-PL-DHA and dose-adherence. Bayesian hierarchical models were also fitted for EPTB by dose adherence and quartiles of baseline RBC-PL-DHA.
Results: Adherence to the high dose using both RBC-PL-DHA cut points resulted in less EPTB compared to 200 mg [Bayesian posterior probability (pp) = 0.93 and 0.92, respectively]. For participants in the two lowest quartiles of baseline DHA status, adherence to the higher dose resulted in lower EPTB (≥5.5% RBC-PL-DHA, quartiles 1 and 2, pp = 0.95 and 0.96; ≥8% RBC-PL-DHA, quartiles 1 and 2, pp = 0.94 and 0.95). Using the Bayesian model, EPTB was reduced by 65%, from 3.45% to 1.2%, using both cut points. Adherence also reduced PTB before 35, 36 and 37 weeks using both cut points (pp ≥ 0.95). In general, performance of the nonadherent subgroup mirrored that of participants assigned to 200 mg.
Conclusion: Adherence to high dose DHA reduced EPTB and PTB. The largest effect of adherence on reducing EPTB was observed in women with low baseline DHA levels.
Clinicaltrials: gov (NCT02626299).
Keywords: Clinical trial adherence; DHA; Pregnancy; Preterm birth; Red blood cell phospholipid DHA.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest SEC has received honorariums for presentations about DHA in infancy and pregnancy. SEC, BJG and CJV were PIs of R01HD083292, CJV was an employee of RB Nutrition, which produces infant formulas and supplements with DHA at the time the study was conducted, however, RB was not involved in the study execution or analysis. She conducted this study through her role as an Adjunct Professor at The University of Cincinnati. The other authors have no competing interests.
Figures
Similar articles
-
Assessment of DHA on reducing early preterm birth: the ADORE randomized controlled trial protocol.BMC Pregnancy Childbirth. 2017 Feb 13;17(1):62. doi: 10.1186/s12884-017-1244-5. BMC Pregnancy Childbirth. 2017. PMID: 28193189 Free PMC article. Clinical Trial.
-
DHA supplementation for early preterm birth prevention: An application of Bayesian finite mixture models to adaptive clinical trial design optimization.Contemp Clin Trials. 2024 Sep;144:107633. doi: 10.1016/j.cct.2024.107633. Epub 2024 Jul 14. Contemp Clin Trials. 2024. PMID: 39013543
-
Docosahexaenoic acid (DHA) intake estimated from a 7-question survey identifies pregnancies most likely to benefit from high-dose DHA supplementation.Clin Nutr ESPEN. 2023 Feb;53:93-99. doi: 10.1016/j.clnesp.2022.12.004. Epub 2022 Dec 6. Clin Nutr ESPEN. 2023. PMID: 36657936 Free PMC article. Clinical Trial.
-
Prenatal Nutritional Strategies to Reduce the Risk of Preterm Birth.Ann Nutr Metab. 2020;76 Suppl 3:31-39. doi: 10.1159/000509901. Epub 2021 Jan 19. Ann Nutr Metab. 2020. PMID: 33465767 Review.
-
Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review.Evid Rep Technol Assess (Full Rep). 2016 Oct;(224):1-826. doi: 10.23970/AHRQEPCERTA224. Evid Rep Technol Assess (Full Rep). 2016. PMID: 30307735
Cited by
-
DHA, nutrient intake, and maternal characteristics as predictors of pregnancy outcomes in a randomised clinical trial of DHA supplementation.Clin Nutr. 2023 Nov;42(11):2229-2240. doi: 10.1016/j.clnu.2023.09.005. Epub 2023 Sep 20. Clin Nutr. 2023. PMID: 37806075 Free PMC article. Clinical Trial.
-
Enhancing DHA supplementation adherence: A Bayesian approach with finite mixture models and irregular interim schedules in adaptive trial designs.Stat Methods Med Res. 2024 Nov;33(11-12):2062-2078. doi: 10.1177/09622802241283165. Epub 2024 Oct 4. Stat Methods Med Res. 2024. PMID: 39363807
-
Racial disparity in efficacy of docosahexaenoic acid supplementation for prevention of preterm birth: secondary analysis from a randomized, double-blind trial.Am J Obstet Gynecol MFM. 2024 May;6(5):101358. doi: 10.1016/j.ajogmf.2024.101358. Epub 2024 Mar 27. Am J Obstet Gynecol MFM. 2024. PMID: 38552960 Free PMC article. Clinical Trial. No abstract available.
-
Predictors of compliance with higher dose omega-3 fatty acid supplementation during pregnancy and implications for the risk of prematurity: exploratory analysis of the ORIP randomised trial.BMJ Open. 2023 Sep 22;13(9):e076507. doi: 10.1136/bmjopen-2023-076507. BMJ Open. 2023. PMID: 37739459 Free PMC article. Clinical Trial.
-
Dietary Implications of Polyunsaturated Fatty Acids during Pregnancy and in Neonates.Life (Basel). 2023 Jul 29;13(8):1656. doi: 10.3390/life13081656. Life (Basel). 2023. PMID: 37629513 Free PMC article. Review.
References
-
- Simmonds LA, Sullivan TR, Skubisz M, Middleton PF, Best KP, Yelland LN, et al. Omega-3 fatty acid supplementation in pregnancy – baseline omega-3 status and early preterm birth: exploratory analysis of a randomised controlled trial. BJOG Int J Obstet Gynaecol 2020;127(8):975–81. doi: 10.1111/1471-0528.16168 - DOI - PubMed
-
- Carlson SE, Gajewski BJ, Valentine CJ, Kerling EH, Weinger CP, Cackovic M, Buhimschi CS, Rogers LK, Sands SA, Brown AR, Mudaranthakam DP, Crawford SA, DeFranco EA. Higher dose docosahexaenoic acid supplementation during pregnancy and early preterm birth: A randomised, double-blind, adaptive-design superiority trial. EClinMed 2021; 36:100905 doi: 10.1016/j.eclinm.2021.100905. eCollection 2021 Jun. - DOI - PMC - PubMed
-
- Food and Agriculture Organization of the United Nations. Fats and fatty acids in human nutrition: Report of an expert consultation. FAO Food and Nutrition paper 91. Rome 2010. Available at http://www.fao.org/docrep/013/i1953e/i1953e00.pdf - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

