Red blood cells from endothelial nitric oxide synthase-deficient mice induce vascular dysfunction involving oxidative stress and endothelial arginase I
- PMID: 36681048
- PMCID: PMC9868875
- DOI: 10.1016/j.redox.2023.102612
Red blood cells from endothelial nitric oxide synthase-deficient mice induce vascular dysfunction involving oxidative stress and endothelial arginase I
Abstract
Background & aims: Nitric oxide bioactivity (NO) from endothelial NO synthase (eNOS) importantly contributes to the maintenance of vascular homeostasis, and reduced eNOS activity has been associated with cardiovascular disease. Emerging evidence suggests interaction(s) between red blood cells (RBCs) and the endothelium in vascular control; however, the specific role of RBC eNOS is less clear. We aimed to investigate the hypothesis that a lack of RBC eNOS induces endothelial dysfunction.
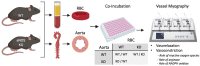
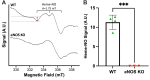
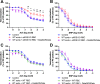
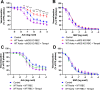
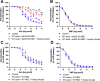
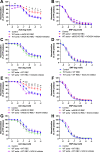
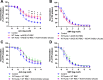
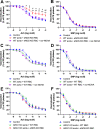
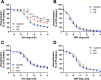
Methods & results: RBCs from global eNOS knockout (KO) and wildtype (WT) mice were co-incubated ex vivo overnight with healthy mouse aortic rings, followed by functional and mechanistic analyses of endothelium-dependent and independent relaxations. RBCs from eNOS KO mice induced endothelial dysfunction and vascular oxidative stress, whereas WT RBC did not. No differences were observed for endothelium-independent relaxations. This eNOS KO RBC-induced endothelial dysfunctional phenotype was prevented by concomitant co-incubation with reactive oxygen species scavenger (TEMPOL), arginase inhibitor (nor-NOHA), NO donor (detaNONOate) and NADPH oxidase 4 (NOX4) inhibitor. Moreover, vessels from endothelial cell-specific arginase 1 KO mice were resistant to eNOS KO-RBC-induced endothelial dysfunction. Finally, in mice aortae co-incubated with RBCs from women with preeclampsia, we observed a significant reduction in endothelial function compared to when using RBCs from healthy pregnant women or from women with uncomplicated gestational hypertension.
Conclusions: RBCs from mice lacking eNOS, and patients with preeclampsia, induce endothelial dysfunction in adjacent blood vessels. Thus, RBC-derived NO bioactivity acts to prevent induction of vascular oxidative stress occurring via RBC NOX4-derived ROS in a vascular arginase-dependent manner. Our data highlight the intrinsic protective role of RBC-derived NO bioactivity in preventing the damaging potential of RBCs. This provides novel insight into the functional relationship between RBCs and the vasculature in health and cardiovascular disease, including preeclampsia.
Keywords: Arginase; Nitric oxide; Oxidative stress; Red blood cells; eNOS.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Lundberg J.O., Weitzberg E. Nitric oxide signaling in health and disease. Cell. 2022;185(16):2853–2878. - PubMed
-
- Lundberg J.O., Gladwin M.T., Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015;14(9):623–641. - PubMed
-
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993;329(27):2002–2012. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

