Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression
- PMID: 36681084
- PMCID: PMC10014083
- DOI: 10.1016/S1473-3099(22)00801-5
Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression
Abstract
Background: The global surge in the omicron (B.1.1.529) variant has resulted in many individuals with hybrid immunity (immunity developed through a combination of SARS-CoV-2 infection and vaccination). We aimed to systematically review the magnitude and duration of the protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against infection and severe disease caused by the omicron variant.
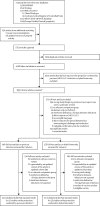
Methods: For this systematic review and meta-regression, we searched for cohort, cross-sectional, and case-control studies in MEDLINE, Embase, Web of Science, ClinicalTrials.gov, the Cochrane Central Register of Controlled Trials, the WHO COVID-19 database, and Europe PubMed Central from Jan 1, 2020, to June 1, 2022, using keywords related to SARS-CoV-2, reinfection, protective effectiveness, previous infection, presence of antibodies, and hybrid immunity. The main outcomes were the protective effectiveness against reinfection and against hospital admission or severe disease of hybrid immunity, hybrid immunity relative to previous infection alone, hybrid immunity relative to previous vaccination alone, and hybrid immunity relative to hybrid immunity with fewer vaccine doses. Risk of bias was assessed with the Risk of Bias In Non-Randomized Studies of Interventions Tool. We used log-odds random-effects meta-regression to estimate the magnitude of protection at 1-month intervals. This study was registered with PROSPERO (CRD42022318605).
Findings: 11 studies reporting the protective effectiveness of previous SARS-CoV-2 infection and 15 studies reporting the protective effectiveness of hybrid immunity were included. For previous infection, there were 97 estimates (27 with a moderate risk of bias and 70 with a serious risk of bias). The effectiveness of previous infection against hospital admission or severe disease was 74·6% (95% CI 63·1-83·5) at 12 months. The effectiveness of previous infection against reinfection waned to 24·7% (95% CI 16·4-35·5) at 12 months. For hybrid immunity, there were 153 estimates (78 with a moderate risk of bias and 75 with a serious risk of bias). The effectiveness of hybrid immunity against hospital admission or severe disease was 97·4% (95% CI 91·4-99·2) at 12 months with primary series vaccination and 95·3% (81·9-98·9) at 6 months with the first booster vaccination after the most recent infection or vaccination. Against reinfection, the effectiveness of hybrid immunity following primary series vaccination waned to 41·8% (95% CI 31·5-52·8) at 12 months, while the effectiveness of hybrid immunity following first booster vaccination waned to 46·5% (36·0-57·3) at 6 months.
Interpretation: All estimates of protection waned within months against reinfection but remained high and sustained for hospital admission or severe disease. Individuals with hybrid immunity had the highest magnitude and durability of protection, and as a result might be able to extend the period before booster vaccinations are needed compared to individuals who have never been infected.
Funding: WHO COVID-19 Solidarity Response Fund and the Coalition for Epidemic Preparedness Innovations.
© 2023 World Health Organization; licensee Elsevier. This is an Open Access article published under the CC BY 3.0 IGO license which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any use of this article, there should be no suggestion that WHO endorses any specific organisation, products or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Conflict of interest statement
Declaration of interests NB, HW, XM, ZL, RH, CC, AS, MW, BC, and RKA report grants unrelated to the present work from the WHO Health Emergencies Program, the Public Health Agency of Canada through Canada's COVID-19 Immunity Task Force (grant 2021-HQ-000056), the Robert Koch Institute, and the Canadian Medical Association Joule Innovation Fund. HI reports contracts unrelated to the present work from WHO. MMH reports contracts unrelated to the present work from WHO, Pfizer, and the Bill & Melinda Gates Foundation. VP is an unpaid governing board member and Royal Trustee at Cochrane. RKA reports consulting fees unrelated to the present work from the Bill & Melinda Gates Foundation Strategic Investment Fund, Health Canada, and Flagship Pioneering, and stock in Alethea Medical that is unrelated to the present work. MDVK, IB, DF, and LS are employed by WHO. MMH has contracts with WHO, CEPI, and the Asian Development Bank to gather data on COVID-19 vaccine efficacy, effectiveness, and safety from published studies, and reports unrelated grants from Pfizer and the Bill & Melinda Gates Foundation. AW-S and MKP consult on COVID-19 research and policy for WHO.
Figures


Comment in
-
The benefit of vaccination after previous SARS-CoV-2 infection in the omicron era.Lancet Infect Dis. 2023 May;23(5):511-512. doi: 10.1016/S1473-3099(22)00880-5. Epub 2023 Jan 18. Lancet Infect Dis. 2023. PMID: 36681085 Free PMC article. No abstract available.
-
Risk of omicron infection for high-risk older adults in long-term care facilities.Lancet Infect Dis. 2023 May;23(5):526-527. doi: 10.1016/S1473-3099(23)00179-2. Epub 2023 Mar 17. Lancet Infect Dis. 2023. PMID: 36940702 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

