Islet autoantibody screening in at-risk adolescents to predict type 1 diabetes until young adulthood: a prospective cohort study
- PMID: 36681087
- PMCID: PMC10038928
- DOI: 10.1016/S2352-4642(22)00350-9
Islet autoantibody screening in at-risk adolescents to predict type 1 diabetes until young adulthood: a prospective cohort study
Abstract
Background: Screening for islet autoantibodies in children and adolescents identifies individuals who will later develop type 1 diabetes, allowing patient and family education to prevent diabetic ketoacidosis at onset and to enable consideration of preventive therapies. We aimed to assess whether islet autoantibody screening is effective for predicting type 1 diabetes in adolescents aged 10-18 years with an increased risk of developing type 1 diabetes.
Methods: Data were harmonised from prospective studies from Finland (the Diabetes Prediction and Prevention study), Germany (the BABYDIAB study), and the USA (Diabetes Autoimmunity Study in the Young and the Diabetes Evaluation in Washington study). Autoantibodies against insulin, glutamic acid decarboxylase, and insulinoma-associated protein 2 were measured at each follow-up visit. Children who were lost to follow-up or diagnosed with type 1 diabetes before 10 years of age were excluded. Inverse probability censoring weighting was used to include data from remaining participants. Sensitivity and the positive predictive value of these autoantibodies, tested at one or two ages, to predict type 1 diabetes by the age of 18 years were the main outcomes.
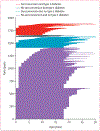
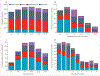
Findings: Of 20 303 children with an increased type 1 diabetes risk, 8682 were included for the analysis with inverse probability censoring weighting. 1890 were followed up to 18 years of age or developed type 1 diabetes between the ages of 10 years and 18 years, and their median follow-up was 18·3 years (IQR 14·5-20·3). 442 (23·4%) of 1890 adolescents were positive for at least one islet autoantibody, and 262 (13·9%) developed type 1 diabetes. Time from seroconversion to diabetes diagnosis increased by 0·64 years (95% CI 0·34-0·95) for each 1-year increment of diagnosis age (Pearson's correlation coefficient 0·88, 95% CI 0·50-0·97, p=0·0020). The median interval between the last prediagnostic sample and diagnosis was 0·3 years (IQR 0·1-1·3) in the 227 participants who were autoantibody positive and 6·8 years (1·6-9·9) for the 35 who were autoantibody negative. Single screening at the age of 10 years was 90% (95% CI 86-95) sensitive, with a positive predictive value of 66% (60-72) for clinical diabetes. Screening at two ages (10 years and 14 years) increased sensitivity to 93% (95% CI 89-97) but lowered the positive predictive value to 55% (49-60).
Interpretation: Screening of adolescents at risk for type 1 diabetes only once at 10 years of age for islet autoantibodies was highly effective to detect type 1 diabetes by the age of 18 years, which in turn could enable prevention of diabetic ketoacidosis and participation in secondary prevention trials.
Funding: JDRF International.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MG and VA are employees of IBM. FM and OL performed this work as employees of JDRF. All other authors declare no competing interests.
Figures


Comment in
-
Paving the way for evidence-based population screening for type 1 diabetes.Lancet Child Adolesc Health. 2023 Apr;7(4):225-226. doi: 10.1016/S2352-4642(23)00001-9. Epub 2023 Jan 18. Lancet Child Adolesc Health. 2023. PMID: 36681088 No abstract available.
References
-
- Barker JM, Barriga KJ, Yu L, et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004; 89: 3896–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

