Scavenger receptor BI attenuates oxidized phospholipid-induced pulmonary inflammation
- PMID: 36681128
- PMCID: PMC9983330
- DOI: 10.1016/j.taap.2023.116381
Scavenger receptor BI attenuates oxidized phospholipid-induced pulmonary inflammation
Abstract
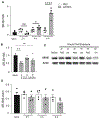
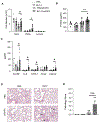
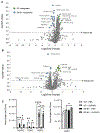
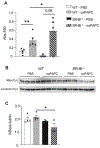
Damage associated molecular patterns (DAMPs) are molecules released from dead/dying cells following toxicant and/or environmental exposures that activate the immune response through binding of pattern recognition receptors (PRRs). Excessive production of DAMPs or failed clearance leads to chronic inflammation and delayed inflammation resolution. One category of DAMPs are oxidized phospholipids (oxPLs) produced upon exposure to high levels of oxidative stress, such as following ozone (O3) induced inflammation. OxPLs are bound by multiple classes of PRRs that include scavenger receptors (SRs) such as SR class B-1 (SR-BI) and toll-like receptors (TLRs). Interactions between oxPLs and PRRs appear to regulate inflammation; however, the role of SR-BI in oxPL-induced lung inflammation has not been defined. Therefore, we hypothesize that SR-BI is critical in protecting the lung from oxPL-induced pulmonary inflammation/injury. To test this hypothesis, C57BL/6J (WT) female mice were dosed with oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylcholine (oxPAPC) by oropharyngeal aspiration which increased pulmonary SR-BI expression. Following oxPAPC exposure, SR-BI deficient (SR-BI-/-) mice exhibited increased lung pathology and inflammatory cytokine/chemokine production. Lipidomic analysis revealed that SR-BI-/- mice had an altered pulmonary lipidome prior to and following oxPAPC exposure, which correlated with increased oxidized phosphatidylcholines (PCs). Finally, we characterized TLR4-mediated activation of NF-κB following oxPAPC exposure and discovered that SR-BI-/- mice had increased TLR4 mRNA expression in lung tissue and macrophages, increased nuclear p65, and decreased cytoplasmic IκBα. Overall, we conclude that SR-BI is required for limiting oxPAPC-induced lung pathology by maintaining lipid homeostasis, reducing oxidized PCs, and attenuating TLR4-NF-κB activation, thereby preventing excessive and persistent inflammation.
Keywords: DAMP; Inflammation; Lung; SR-BI; TLR4.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

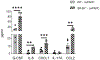
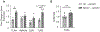
References
-
- Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112. - PubMed
-
- Stamenkovic A, Pierce GN, Ravandi A. Oxidized lipids: not just another brick in the wall (1). Can J Physiol Pharmacol. 2019;97(6):473–85. - PubMed
-
- Minagawa S, Yoshida M, Araya J, Hara H, Imai H, Kuwano K. Regulated Necrosis in Pulmonary Disease. A Focus on Necroptosis and Ferroptosis. Am J Respir Cell Mol Biol. 2020;62(5):554–62. - PubMed
-
- Kuwano K, Kunitake R, Kawasaki M, Nomoto Y, Hagimoto N, Nakanishi Y, et al. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154(2 Pt 1):477–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

