Resveratrol protects osteocytes against oxidative stress in ovariectomized rats through AMPK/JNK1-dependent pathway leading to promotion of autophagy and inhibition of apoptosis
- PMID: 36681672
- PMCID: PMC9867734
- DOI: 10.1038/s41420-023-01331-2
Resveratrol protects osteocytes against oxidative stress in ovariectomized rats through AMPK/JNK1-dependent pathway leading to promotion of autophagy and inhibition of apoptosis
Abstract
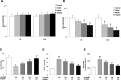
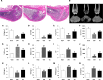
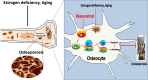
A large number of studies in recent years indicate that osteocytes are the orchestrators of bone remodeling by regulating both osteoblast and osteoclast activities. Oxidative stress-induced osteocyte apoptosis plays critical roles in the pathological processes of postmenopausal osteoporosis. Resveratrol is a natural polyphenolic compound that ameliorates postmenopausal osteoporosis. However, whether resveratrol regulates osteocyte apoptosis via autophagy remains largely unknown. The effects of resveratrol on regulating osteocyte apoptosis and autophagy were analyzed both in vivo and in vitro. In vitro, cultured MLO-Y4 cells were exposed to H2O2 with or without resveratrol. In vivo, an ovariectomy-induced osteoporosis model was constructed in rats with or without daily intraperitoneal injection of 10 mg/kg body weight resveratrol. It was found that resveratrol attenuated H2O2-induced apoptosis through activating autophagy in cultured MLO-Y4 cells, which was mediated by the dissociation of Beclin-1/Bcl-2 complex in AMPK/JNK1-dependent pathway, ultimately regulating osteocytes function. Furthermore, it was shown that resveratrol treatment reduced osteocytes oxidative stress, inhibited osteocytes apoptosis and promoted autophagy in ovariectomized rats. Our study suggests that resveratrol protects against oxidative stress by restoring osteocytes autophagy and alleviating apoptosis via AMPK/JNK1 activation, therefore dissociating Bcl-2 from Beclin-1.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Farr JN, Rowsey JL, Eckhardt BA, Thicke BS, Fraser DG, Tchkonia T, et al. Independent roles of estrogen deficiency and cellular senescence in the pathogenesis of osteoporosis: evidence in young adult mice and older humans. J Bone Miner Res. 2019;34:1407–18. doi: 10.1002/jbmr.3729. - DOI - PMC - PubMed
-
- Watson SL, Weeks BK, Weis LJ, Harding AT, Horan SA, Beck BR. High-intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: The LIFTMOR randomized controlled trial. J Bone Miner Res. 2018;33:211–20. doi: 10.1002/jbmr.3284. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

