A microfluidic-based PDAC organoid system reveals the impact of hypoxia in response to treatment
- PMID: 36681673
- PMCID: PMC9867742
- DOI: 10.1038/s41420-023-01334-z
A microfluidic-based PDAC organoid system reveals the impact of hypoxia in response to treatment
Abstract
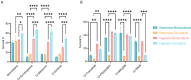
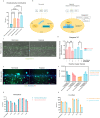
Pancreatic Ductal Adenocarcinoma (PDAC) is estimated to become the second leading cause of cancer-related deaths by 2030 with mortality rates of up to 93%. Standard of care chemotherapeutic treatment only prolongs the survival of patients for a short timeframe. Therefore, it is important to understand events driving treatment failure in PDAC as well as identify potential more effective treatment opportunities. PDAC is characterized by a high-density stroma, high interstitial pressure and very low oxygen tension. The aim of this study was to establish a PDAC platform that supported the understanding of treatment response of PDAC organoids in mono-, and co-culture with pancreatic stellate cells (PSCs) under hypoxic and normoxic conditions. Cultures were exposed to Gemcitabine in combination with molecules targeting relevant molecular programs that could explain treatment specific responses under different oxygen pressure conditions. Two groups of treatment responses were identified, showing either a better effect in monoculture or co-culture. Moreover, treatment response also differed between normoxia and hypoxia. Modulation of response to Gemcitabine was also observed in presence of a Hypoxia-inducible factor (HIF) prolyl hydroxylase (PHD) inhibitor and HIF inhibitors. Altogether this highlights the importance of adjusting experimental conditions to include relevant oxygen levels in drug response studies in PDAC.
© 2023. The Author(s).
Conflict of interest statement
MG, LG and KQ are employees of MIMETAS BV, the Netherlands, which is marketing the OrganoPlate®.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

