Application of metabolomics in urolithiasis: the discovery and usage of succinate
- PMID: 36681678
- PMCID: PMC9867757
- DOI: 10.1038/s41392-023-01311-z
Application of metabolomics in urolithiasis: the discovery and usage of succinate
Abstract
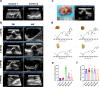
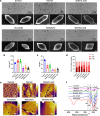
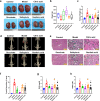
Urinary stone is conceptualized as a chronic metabolic disorder punctuated by symptomatic stone events. It has been shown that the occurrence of calcium oxalate monohydrate (COM) during stone formation is regulated by crystal growth modifiers. Although crystallization inhibitors have been recognized as a therapeutic modality for decades, limited progress has been made in the discovery of effective modifiers to intervene with stone disease. In this study, we have used metabolomics technologies, a powerful approach to identify biomarkers by screening the urine components of the dynamic progression in a bladder stone model. By in-depth mining and analysis of metabolomics data, we have screened five differential metabolites. Through density functional theory studies and bulk crystallization, we found that three of them (salicyluric, gentisic acid and succinate) could effectively inhibit nucleation in vitro. We thereby assessed the impact of the inhibitors with an EG-induced rat model for kidney stones. Notably, succinate, a key player in the tricarboxylic acid cycle, could decrease kidney calcium deposition and injury in the model. Transcriptomic analysis further showed that the protective effect of succinate was mainly through anti-inflammation, inhibition of cell adhesion and osteogenic differentiation. These findings indicated that succinate may provide a new therapeutic option for urinary stones.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Peeping into human renal calcium oxalate stone matrix: characterization of novel proteins involved in the intricate mechanism of urolithiasis.PLoS One. 2013 Jul 24;8(7):e69916. doi: 10.1371/journal.pone.0069916. Print 2013. PLoS One. 2013. PMID: 23894559 Free PMC article.
-
Idiopathic recurrent calcium urolithiasis (IRCU): variation of fasting urinary protein is a window to pathophysiology or simple consequence of renal stones in situ? A tripartite study in male patients providing insight into oxidative metabolism as possible driving force towards alteration of urine composition, calcium salt crystallization and stone formation.Eur J Med Res. 2009 Sep 1;14(9):378-92. doi: 10.1186/2047-783x-14-9-378. Eur J Med Res. 2009. PMID: 19748857 Free PMC article.
-
Molecular modifiers reveal a mechanism of pathological crystal growth inhibition.Nature. 2016 Aug 25;536(7617):446-50. doi: 10.1038/nature19062. Epub 2016 Aug 17. Nature. 2016. PMID: 27501150
-
[Molecular mechanism of renal stone formation].Clin Calcium. 2011 Oct;21(10):1481-7. Clin Calcium. 2011. PMID: 21960233 Review. Japanese.
-
The advances of calcium oxalate calculi associated drugs and targets.Eur J Pharmacol. 2022 Nov 15;935:175324. doi: 10.1016/j.ejphar.2022.175324. Epub 2022 Oct 17. Eur J Pharmacol. 2022. PMID: 36257382 Review.
Cited by
-
Regulating the balance between GSDMD-mediated pyroptosis and CHMP4B-dependent cell repair attenuates calcium oxalate kidney stone formation.Int J Biol Sci. 2025 Apr 22;21(7):3099-3121. doi: 10.7150/ijbs.105415. eCollection 2025. Int J Biol Sci. 2025. PMID: 40384863 Free PMC article.
-
Mendelian randomization study of urolithiasis: exploration of risk factors using human blood metabolites.BMC Urol. 2024 Aug 28;24(1):182. doi: 10.1186/s12894-024-01568-8. BMC Urol. 2024. PMID: 39198784 Free PMC article.
-
Identification of the relationship between 1400 blood metabolites and urolithiasis: A bidirectional Mendelian randomization study.Medicine (Baltimore). 2025 Mar 21;104(12):e41911. doi: 10.1097/MD.0000000000041911. Medicine (Baltimore). 2025. PMID: 40128055 Free PMC article.
-
Serum metabolomics study reveals a distinct metabolic diagnostic model for renal calculi.Heliyon. 2024 Jun 8;10(11):e32482. doi: 10.1016/j.heliyon.2024.e32482. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38912451 Free PMC article.
-
A New Perspective on Gas Chromatography-Mass Spectrometry Urinary Metabolomic Analysis and Efficient Risk Assessment of Urolithiasis: Morning Urine Organic Acid Profiles.Kidney Blood Press Res. 2025;50(1):83-96. doi: 10.1159/000542263. Epub 2024 Dec 11. Kidney Blood Press Res. 2025. PMID: 39662072 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

