Identification of platelet subpopulations in cryopreserved platelet components using multi-colour imaging flow cytometry
- PMID: 36681723
- PMCID: PMC9867743
- DOI: 10.1038/s41598-023-28352-2
Identification of platelet subpopulations in cryopreserved platelet components using multi-colour imaging flow cytometry
Abstract
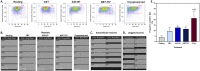
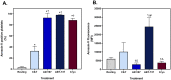
Cryopreservation of platelets, at - 80 °C with 5-6% DMSO, results in externalisation of phosphatidylserine and the formation of extracellular vesicles (EVs), which may mediate their procoagulant function. The phenotypic features of procoagulant platelets overlap with other platelet subpopulations. The aim of this study was to define the phenotype of in vitro generated platelet subpopulations, and subsequently identify the subpopulations present in cryopreserved components. Fresh platelet components (n = 6 in each group) were either unstimulated as a source of resting platelets; or stimulated with thrombin and collagen to generate a mixture of aggregatory and procoagulant platelets; calcium ionophore (A23187) to generate procoagulant platelets; or ABT-737 to generate apoptotic platelets. Platelet components (n = 6) were cryopreserved with DMSO, thawed and resuspended in a unit of thawed plasma. Multi-colour panels of fluorescent antibodies and dyes were used to identify the features of subpopulations by imaging flow cytometry. A combination of annexin-V (AnnV), CD42b, and either PAC1 or CD62P was able to distinguish the four subpopulations. Cryopreserved platelets contained procoagulant platelets (AnnV+/PAC1-/CD42b+/CD62P+) and a novel population (AnnV+/PAC1-/CD42b+/CD62P-) that did not align with the phenotype of aggregatory (AnnV-/PAC1+/CD42b+/CD62P+) or apoptotic (AnnV+/PAC1-/CD42b-/CD62P-) subpopulations. These data suggests that the enhanced haemostatic potential of cryopreserved platelets may be due to the cryo-induced development of procoagulant platelets, and that additional subpopulations may exist.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The phenotype of cryopreserved platelets influences the formation of platelet-leukocyte aggregates in an in vitro model.Platelets. 2023 Dec;34(1):2206916. doi: 10.1080/09537104.2023.2206916. Platelets. 2023. PMID: 37143347
-
[Protection of cryopreserved platelets by dimethyl sulfoxide combined with trehalose].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009 Oct;17(5):1373-9. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009. PMID: 19840487 Chinese.
-
Multidimensional flow cytometry reveals novel platelet subpopulations in response to prostacyclin.J Thromb Haemost. 2021 Jul;19(7):1800-1812. doi: 10.1111/jth.15330. Epub 2021 Apr 26. J Thromb Haemost. 2021. PMID: 33834609
-
Review of in vivo studies of dimethyl sulfoxide cryopreserved platelets.Transfus Med Rev. 2014 Oct;28(4):212-25. doi: 10.1016/j.tmrv.2014.09.001. Epub 2014 Sep 21. Transfus Med Rev. 2014. PMID: 25439164 Review.
-
Platelet Biochemistry and Morphology after Cryopreservation.Int J Mol Sci. 2020 Jan 31;21(3):935. doi: 10.3390/ijms21030935. Int J Mol Sci. 2020. PMID: 32023815 Free PMC article. Review.
Cited by
-
Western Diet Modifies Platelet Activation Profiles in Male Mice.Int J Mol Sci. 2024 Jul 23;25(15):8019. doi: 10.3390/ijms25158019. Int J Mol Sci. 2024. PMID: 39125586 Free PMC article.
-
Storage Temperature Affects Platelet Activation and Degranulation in Response to Stimuli.Int J Mol Sci. 2025 Mar 24;26(7):2944. doi: 10.3390/ijms26072944. Int J Mol Sci. 2025. PMID: 40243579 Free PMC article.
-
The Effect of Leukocyte Removal and Matrix Metalloproteinase Inhibition on Platelet Storage Lesions.Cells. 2024 Mar 13;13(6):506. doi: 10.3390/cells13060506. Cells. 2024. PMID: 38534349 Free PMC article.
-
Cryopreserved Platelets in a Non-Toxic DMSO-Free Solution Maintain Hemostatic Function In Vitro.Int J Mol Sci. 2023 Aug 23;24(17):13097. doi: 10.3390/ijms241713097. Int J Mol Sci. 2023. PMID: 37685902 Free PMC article.
-
Heterogeneity of platelets and their responses.Res Pract Thromb Haemost. 2024 Mar 1;8(2):102356. doi: 10.1016/j.rpth.2024.102356. eCollection 2024 Feb. Res Pract Thromb Haemost. 2024. PMID: 38666061 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

