Cholinergic system adaptations are associated with cognitive function in people recently abstinent from smoking: a (-)-[18F]flubatine PET study
- PMID: 36681758
- PMCID: PMC9938267
- DOI: 10.1038/s41386-023-01535-1
Cholinergic system adaptations are associated with cognitive function in people recently abstinent from smoking: a (-)-[18F]flubatine PET study
Abstract
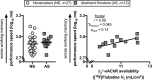
The cholinergic system is a critical mediator of cognition in animals. People who smoke cigarettes exhibit cognitive deficits, especially during quit attempts. Few studies jointly examine the cholinergic system and cognition in people while trying to quit smoking. We used positron emission tomography (PET) brain imaging with the β2-subunit containing nicotinic acetylcholine receptor (β2*-nAChR) partial agonist radioligand (-)-[18F]flubatine and the acetylcholinesterase inhibitor physostigmine to jointly examine the cholinergic system, smoking status, and cognition. (-)-[18F]Flubatine scans and cognitive data were acquired from twenty people who recently stopped smoking cigarettes (aged 38 ± 11 years; 6 female, 14 male; abstinent 7 ± 1 days) and 27 people who never smoked cigarettes (aged 29 ± 8 years; 11 female, 16 male). A subset of fifteen recently abstinent smokers and 21 never smokers received a mid-scan physostigmine challenge to increase acetylcholine levels. Regional volume of distribution (VT) was estimated with equilibrium analysis at "baseline" and post-physostigmine. Participants completed a cognitive battery prior to (-)-[18F]flubatine injection and physostigmine administration assessing executive function (Groton Maze Learning test), verbal learning (International Shopping List test), and working memory (One Back test). Physostigmine significantly decreased cortical (-)-[18F]flubatine VT, consistent with increased cortical acetylcholine levels reducing the number of β2*-nAChR sites available for (-)-[18F]flubatine binding, at comparable magnitudes across groups (p values < 0.05). A larger magnitude of physostigmine-induced decrease in (-)-[18F]flubatine VT was significantly associated with worse executive function in people who recently stopped smoking (p values < 0.05). These findings underscore the role of the cholinergic system in early smoking cessation and highlight the importance of neuroscience-informed treatment strategies.
© 2023. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Imaging of cerebral α4β2* nicotinic acetylcholine receptors with (-)-[(18)F]Flubatine PET: Implementation of bolus plus constant infusion and sensitivity to acetylcholine in human brain.Neuroimage. 2016 Nov 1;141:71-80. doi: 10.1016/j.neuroimage.2016.07.026. Epub 2016 Jul 15. Neuroimage. 2016. PMID: 27426839 Free PMC article.
-
Cognitive correlates of α4β2 nicotinic acetylcholine receptors in mild Alzheimer's dementia.Brain. 2018 Jun 1;141(6):1840-1854. doi: 10.1093/brain/awy099. Brain. 2018. PMID: 29672680 Free PMC article.
-
Evaluation of (-)-[18 F]Flubatine-specific binding: Implications for reference region approaches.Synapse. 2018 Mar;72(3):10.1002/syn.22016. doi: 10.1002/syn.22016. Epub 2017 Nov 17. Synapse. 2018. PMID: 29105121 Free PMC article.
-
PET Imaging of Cholinergic Neurotransmission in Neurodegenerative Disorders.J Nucl Med. 2022 Jun;63(Suppl 1):33S-44S. doi: 10.2967/jnumed.121.263198. J Nucl Med. 2022. PMID: 35649648 Review.
-
Radioligand imaging of α4β2* nicotinic acetylcholine receptors in Alzheimer's disease and Parkinson's disease.Q J Nucl Med Mol Imaging. 2014 Dec;58(4):376-86. Epub 2014 Nov 11. Q J Nucl Med Mol Imaging. 2014. PMID: 25387119 Review.
Cited by
-
Cholinergic tone abnormalities and relationships with smoking severity in human cigarette smokers: exploratory positron emission tomography study using [18F]VAT.Mol Psychiatry. 2025 Sep;30(9):4064-4071. doi: 10.1038/s41380-025-02985-3. Epub 2025 Mar 31. Mol Psychiatry. 2025. PMID: 40164693
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

