A proteome scale study reveals how plastic surfaces and agitation promote protein aggregation
- PMID: 36681766
- PMCID: PMC9867740
- DOI: 10.1038/s41598-023-28412-7
A proteome scale study reveals how plastic surfaces and agitation promote protein aggregation
Abstract
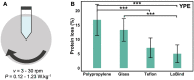
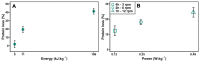
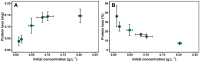
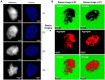
Protein aggregation in biotherapeutics can reduce their activity and effectiveness. It may also promote immune reactions responsible for severe adverse effects. The impact of plastic materials on protein destabilization is not totally understood. Here, we propose to deconvolve the effects of material surface, air/liquid interface, and agitation to decipher their respective role in protein destabilization and aggregation. We analyzed the effect of polypropylene, TEFLON, glass and LOBIND surfaces on the stability of purified proteins (bovine serum albumin, hemoglobin and α-synuclein) and on a cell extract composed of 6000 soluble proteins during agitation (P = 0.1-1.2 W/kg). Proteomic analysis revealed that chaperonins, intrinsically disordered proteins and ribosomes were more sensitive to the combined effects of material surfaces and agitation while small metabolic oligomers could be protected in the same conditions. Protein loss observations coupled to Raman microscopy, dynamic light scattering and proteomic allowed us to propose a mechanistic model of protein destabilization by plastics. Our results suggest that protein loss is not primarily due to the nucleation of small aggregates in solution, but to the destabilization of proteins exposed to material surfaces and their subsequent aggregation at the sheared air/liquid interface, an effect that cannot be prevented by using LOBIND tubes. A guidance can be established on how to minimize these adverse effects. Remove one of the components of this combined stress - material, air (even partially), or agitation - and proteins will be preserved.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Rajan R, et al. Review of the current state of protein aggregation inhibition from a materials chemistry perspective: Special focus on polymeric materials. Mater. Adv. 2021;2:1139–1176. doi: 10.1039/D0MA00760A. - DOI
-
- Vroman L, Adams AL. Findings with the recording ellipsometer suggesting rapid exchange of specific plasma proteins at liquid/solid interfaces. Surf. Sci. 1969;16:438–446. doi: 10.1016/0039-6028(69)90037-5. - DOI
-
- Angioletti-Uberti S, Ballauff M, Dzubiella J. Competitive adsorption of multiple proteins to nanoparticles: The Vroman effect revisited. Mol. Phys. 2018;116:3154–3163. doi: 10.1080/00268976.2018.1467056. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

