MetfOrmin BenefIts Lower Extremities with Intermittent Claudication (MOBILE IC): randomized clinical trial protocol
- PMID: 36681798
- PMCID: PMC9862509
- DOI: 10.1186/s12872-023-03047-8
MetfOrmin BenefIts Lower Extremities with Intermittent Claudication (MOBILE IC): randomized clinical trial protocol
Abstract
Background: Peripheral artery disease (PAD) affects over 230 million people worldwide and is due to systemic atherosclerosis with etiology linked to chronic inflammation, hypertension, and smoking status. PAD is associated with walking impairment and mobility loss as well as a high prevalence of coronary and cerebrovascular disease. Intermittent claudication (IC) is the classic presenting symptom for PAD, although many patients are asymptomatic or have atypical presentations. Few effective medical therapies are available, while surgical and exercise therapies lack durability. Metformin, the most frequently prescribed oral medication for Type 2 diabetes, has salient anti-inflammatory and promitochondrial properties. We hypothesize that metformin will improve function, retard the progression of PAD, and improve systemic inflammation and mitochondrial function in non-diabetic patients with IC.
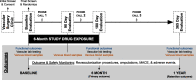
Methods: 200 non-diabetic Veterans with IC will be randomized 1:1 to 180-day treatment with metformin extended release (1000 mg/day) or placebo to evaluate the effect of metformin on functional status, PAD progression, cardiovascular disease events, and systemic inflammation. The primary outcome is 180-day maximum walking distance on the 6-min walk test (6MWT). Secondary outcomes include additional assessments of functional status (cardiopulmonary exercise testing, grip strength, Walking Impairment Questionnaires), health related quality of life (SF-36, VascuQoL), macro- and micro-vascular assessment of lower extremity blood flow (ankle brachial indices, pulse volume recording, EndoPAT), cardiovascular events (amputations, interventions, major adverse cardiac events, all-cause mortality), and measures of systemic inflammation. All outcomes will be assessed at baseline, 90 and 180 days of study drug exposure, and 180 days following cessation of study drug. We will evaluate the primary outcome with linear mixed-effects model analysis with covariate adjustment for baseline 6MWT, age, baseline ankle brachial indices, and smoking status following an intention to treat protocol.
Discussion: MOBILE IC is uniquely suited to evaluate the use of metformin to improve both systematic inflammatory responses, cellular energetics, and functional outcomes in patients with PAD and IC.
Trial registration: The prospective MOBILE IC trial was publicly registered (NCT05132439) November 24, 2021.
Keywords: Anti-inflammatory agents; Atherosclerosis; Clinical trial protocol; Intermittent claudication; Metformin; Peripheral artery disease.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
No author reports any disclosures, conflict of interest or relevant financial interests related to the content of the manuscript. The opinions expressed here are those of the authors and do not necessarily reflect the position of the Department of Veterans Affairs or the US government.
Figures
Similar articles
-
Stenting for peripheral artery disease of the lower extremities: an evidence-based analysis.Ont Health Technol Assess Ser. 2010;10(18):1-88. Epub 2010 Sep 1. Ont Health Technol Assess Ser. 2010. PMID: 23074395 Free PMC article.
-
Effect of ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. Journal of the American Medical Association 2013; 309: 453-460.Vasc Med. 2013 Aug;18(4):234-6. doi: 10.1177/1358863X13497529. Epub 2013 Jul 18. Vasc Med. 2013. PMID: 23867841
-
The efficacy of transcutaneous electrical nerve stimulation on the improvement of walking distance in patients with peripheral arterial disease with intermittent claudication: study protocol for a randomised controlled trial: the TENS-PAD study.Trials. 2017 Aug 10;18(1):373. doi: 10.1186/s13063-017-1997-1. Trials. 2017. PMID: 28797281 Free PMC article. Clinical Trial.
-
Lower Extremity Peripheral Artery Disease Without Chronic Limb-Threatening Ischemia: A Review.JAMA. 2021 Jun 1;325(21):2188-2198. doi: 10.1001/jama.2021.2126. JAMA. 2021. PMID: 34061140 Review.
-
Lack of patient-centered evaluation of outcomes in intermittent claudication literature.J Vasc Surg. 2023 Sep;78(3):828-836. doi: 10.1016/j.jvs.2023.03.497. Epub 2023 Apr 10. J Vasc Surg. 2023. PMID: 37044317 Review.
Cited by
-
View on Metformin: Antidiabetic and Pleiotropic Effects, Pharmacokinetics, Side Effects, and Sex-Related Differences.Pharmaceuticals (Basel). 2024 Apr 8;17(4):478. doi: 10.3390/ph17040478. Pharmaceuticals (Basel). 2024. PMID: 38675438 Free PMC article. Review.
-
Cardiovascular Effectiveness and Safety of Antidiabetic Drugs in Patients with Type 2 Diabetes and Peripheral Artery Disease: Systematic Review.Medicina (Kaunas). 2024 Sep 20;60(9):1542. doi: 10.3390/medicina60091542. Medicina (Kaunas). 2024. PMID: 39336583 Free PMC article.
-
Biomaterial-based vascularization strategies for enhanced treatment of peripheral arterial disease.J Nanobiotechnology. 2025 Feb 12;23(1):103. doi: 10.1186/s12951-025-03140-4. J Nanobiotechnology. 2025. PMID: 39940018 Free PMC article. Review.
-
Strategies to Promote Resiliency: A Randomized Embedded Multifactorial Adaptative Platform (REMAP) Clinical Trial to Study Interventions to Improve Recovery After Surgery in High-Risk Patients.Ann Surg Open. 2025 Apr 2;6(2):e566. doi: 10.1097/AS9.0000000000000566. eCollection 2025 Jun. Ann Surg Open. 2025. PMID: 40557356 Free PMC article.
References
-
- Park SY, Pekas EJ, Headid RJ, Son WM, Wooden TK, Song J, et al. Acute mitochondrial antioxidant intake improves endothelial function, antioxidant enzyme activity, and exercise tolerance in patients with peripheral artery disease. Am J Physiol Hear Circ Physiol. 2020;319:H456–H467. doi: 10.1152/ajpheart.00235.2020. - DOI - PubMed
-
- Mayfield JA, Caps MT, Reiber GE, Maynard C, Czerniecki JM, Sangeorzan BJ. Trends in peripheral vascular procedures in the Veterans Health Administration. J Rehabil Res Dev. 2001;38:1989–1998. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical