Circulating tumor nucleic acids: biology, release mechanisms, and clinical relevance
- PMID: 36681803
- PMCID: PMC9862574
- DOI: 10.1186/s12943-022-01710-w
Circulating tumor nucleic acids: biology, release mechanisms, and clinical relevance
Abstract
Background: Despite advances in early detection and therapies, cancer is still one of the most common causes of death worldwide. Since each tumor is unique, there is a need to implement personalized care and develop robust tools for monitoring treatment response to assess drug efficacy and prevent disease relapse.
Main body: Recent developments in liquid biopsies have enabled real-time noninvasive monitoring of tumor burden through the detection of molecules shed by tumors in the blood. These molecules include circulating tumor nucleic acids (ctNAs), comprising cell-free DNA or RNA molecules passively and/or actively released from tumor cells. Often highlighted for their diagnostic, predictive, and prognostic potential, these biomarkers possess valuable information about tumor characteristics and evolution. While circulating tumor DNA (ctDNA) has been in the spotlight for the last decade, less is known about circulating tumor RNA (ctRNA). There are unanswered questions about why some tumors shed high amounts of ctNAs while others have undetectable levels. Also, there are gaps in our understanding of associations between tumor evolution and ctNA characteristics and shedding kinetics. In this review, we summarize current knowledge about ctNA biology and release mechanisms and put this information into the context of tumor evolution and clinical utility.
Conclusions: A deeper understanding of the biology of ctDNA and ctRNA may inform the use of liquid biopsies in personalized medicine to improve cancer patient outcomes.
Keywords: Biomarkers; Cell-free DNA; Circulating tumor DNA; Circulating tumor RNA; Clinical application; Liquid biopsy; Precision oncology; Shedding mechanisms.
© 2023. The Author(s).
Conflict of interest statement
HG is a stock-holder, board member, and Scientific advisor to ExaiBio Inc. LvtV reports ownership of stocks and part-time employment of Agendia Inc. and is an advisor to ExaiBio Inc. The remaining authors declare no competing interests.
Figures

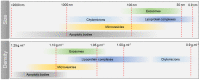
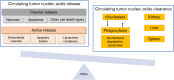
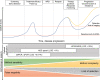
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

