Patient-reported outcome measures for physical function in cancer patients: content comparison of the EORTC CAT Core, EORTC QLQ-C30, SF-36, FACT-G, and PROMIS measures using the International Classification of Functioning, Disability and Health
- PMID: 36681808
- PMCID: PMC9862545
- DOI: 10.1186/s12874-022-01826-z
Patient-reported outcome measures for physical function in cancer patients: content comparison of the EORTC CAT Core, EORTC QLQ-C30, SF-36, FACT-G, and PROMIS measures using the International Classification of Functioning, Disability and Health
Abstract
Background: Patient-reported physical function (PF) is a key endpoint in cancer clinical trials. Using complex statistical methods, common metrics have been developed to compare scores from different patient-reported outcome (PRO) measures, but such methods do not account for possible differences in questionnaire content. Therefore, the aim of our study was a content comparison of frequently used PRO measures for PF in cancer patients.
Methods: Relying on the framework of the International Classification of Functioning, Disability and Health (ICF) we categorized the item content of the physical domains of the following measures: EORTC CAT Core, EORTC QLQ-C30, SF-36, PROMIS Cancer Item Bank for Physical Function, PROMIS Short Form for Physical Function 20a, and the FACT-G. Item content was linked to ICF categories by two independent reviewers.
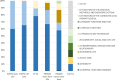
Results: The 118 items investigated were assigned to 3 components ('d - Activities and Participation', 'b - Body Functions', and 'e - Environmental Factors') and 11 first-level ICF categories. All PF items of the EORTC measures but one were assigned to the first-level ICF categories 'd4 - Mobility' and 'd5 - Self-care', all within the component 'd - Activities and Participation'. The SF-36 additionally included item content related to 'd9 - Community, social and civic life' and the PROMIS Short Form for Physical Function 20a also included content related to 'd6 - domestic life'. The PROMIS Cancer Item Bank (v1.1) covered, in addition, two first-level categories within the component 'b - Body Functions'. The FACT-G Physical Well-being scale was found to be the most diverse scale with item content partly not covered by the ICF framework.
Discussion: Our results provide information about conceptual differences between common PRO measures for the assessment of PF in cancer patients. Our results complement quantitative information on psychometric characteristics of these measures and provide a better understanding of the possibilities of establishing common metrics.
Keywords: Cancer; ICF; International classification of functioning disability and health; Linking; PROM; Patient-reported outcome measures; Physical function.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Content comparison of the EORTC CAT Core, SF-36, FACT-G, and PROMIS role and social functioning measures based on the International Classification of Functioning, Disability and Health.Psychooncology. 2023 Sep;32(9):1372-1384. doi: 10.1002/pon.6188. Epub 2023 Jul 25. Psychooncology. 2023. PMID: 37491796
-
Patient-reported outcome measures for emotional functioning in cancer patients: Content comparison of the EORTC CAT Core, FACT-G, HADS, SF-36, PRO-CTCAE, and PROMIS instruments.Psychooncology. 2023 Apr;32(4):628-639. doi: 10.1002/pon.6109. Epub 2023 Feb 19. Psychooncology. 2023. PMID: 36707461
-
Comparing the contents of patient-reported outcome measures for fatigue: EORTC CAT Core, EORTC QLQ-C30, EORTC QLQ-FA12, FACIT, PRO-CTCAE, PROMIS, Brief Fatigue Inventory, Multidimensional Fatigue Inventory, and Piper Fatigue Scale.Health Qual Life Outcomes. 2024 Dec 2;22(1):104. doi: 10.1186/s12955-024-02316-0. Health Qual Life Outcomes. 2024. PMID: 39623483 Free PMC article.
-
Linking assessment instruments for brachial plexus injury to the international classification of functioning, disability and health.J Hand Ther. 2023 Oct-Dec;36(4):885-894. doi: 10.1016/j.jht.2021.04.009. Epub 2021 Apr 14. J Hand Ther. 2023. PMID: 34247880 Review.
-
Outcomes in adults with cerebral palsy: systematic review using the International Classification of Functioning, Disability and Health.Dev Med Child Neurol. 2019 Oct;61(10):1153-1161. doi: 10.1111/dmcn.14247. Epub 2019 Apr 15. Dev Med Child Neurol. 2019. PMID: 30985004
Cited by
-
Evaluating the effect of a mobile-based symptom monitoring system for improving physical function in patients with cancer during chemotherapy: study protocol for a multicentre randomised controlled trial.BMJ Open. 2024 May 1;14(5):e080976. doi: 10.1136/bmjopen-2023-080976. BMJ Open. 2024. PMID: 38692724 Free PMC article.
-
Quality indicators and patient outcome measures for palliative care in cancer patients: a systematic review.Ecancermedicalscience. 2025 Jun 20;19:1929. doi: 10.3332/ecancer.2025.1929. eCollection 2025. Ecancermedicalscience. 2025. PMID: 40606950 Free PMC article. Review.
-
"The Ability to Go Out into the World Is the Most Important Thing"-A Qualitative Study of Important Exercise Outcomes for People with Lung Cancer.Curr Oncol. 2024 Jan 29;31(2):733-746. doi: 10.3390/curroncol31020054. Curr Oncol. 2024. PMID: 38392048 Free PMC article.
-
Physical function endpoints in cancer cachexia clinical trials: Systematic Review 1 of the cachexia endpoints series.J Cachexia Sarcopenia Muscle. 2023 Oct;14(5):1932-1948. doi: 10.1002/jcsm.13321. Epub 2023 Sep 6. J Cachexia Sarcopenia Muscle. 2023. PMID: 37671529 Free PMC article.
-
Patient-reported outcome measures in cancer care: Integration with computerized adaptive testing.Asia Pac J Oncol Nurs. 2023 Oct 27;10(12):100323. doi: 10.1016/j.apjon.2023.100323. eCollection 2023 Dec. Asia Pac J Oncol Nurs. 2023. PMID: 38033390 Free PMC article. No abstract available.
References
-
- Kluetz PG, Slagle A, Papadopoulos EJ, Johnson LL, Donoghue M, Kwitkowski VE, Chen W-H, Sridhara R, Farrell AT, Keegan P, Kim G, Pazdur R. Focusing on Core Patient-Reported Outcomes in Cancer Clinical Trials: Symptomatic Adverse Events, Physical Function, and Disease-Related Symptoms. Clin Cancer Res . 2016;22(7):1553–1558. doi: 10.1158/1078-0432.CCR-15-2035. - DOI - PubMed
-
- European Medicines Agency. (2016). The use of patient-reported outcome (PRO) measures in oncology studies - Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man. https://www.ema.europa.eu/en/documents/other/appendix-2-guideline-evalua...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous