Ketamine and Zinc: Treatment of Anorexia Nervosa Via Dual NMDA Receptor Modulation
- PMID: 36681939
- PMCID: PMC9911496
- DOI: 10.1007/s40263-022-00984-4
Ketamine and Zinc: Treatment of Anorexia Nervosa Via Dual NMDA Receptor Modulation
Abstract
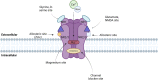
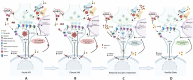
Anorexia nervosa is a disorder associated with serious adverse health outcomes, for which there is currently considerable treatment ineffectiveness. Characterised by restrictive eating behaviours, distorted body image perceptions and excessive physical activity, there is growing recognition anorexia nervosa is associated with underlying dysfunction in excitatory and inhibitory neurometabolite metabolism and signalling. This narrative review critically explores the role of N-methyl-D-aspartate receptor-mediated excitatory and inhibitory neurometabolite dysfunction in anorexia nervosa and its associated biomarkers. The existing magnetic resonance spectroscopy literature in anorexia nervosa is reviewed and we outline the brain region-specific neurometabolite changes that have been reported and their connection to anorexia nervosa psychopathology. Considering the proposed role of dysfunctional neurotransmission in anorexia nervosa, the potential utility of zinc supplementation and sub-anaesthetic doses of ketamine in normalising this is discussed with reference to previous research in anorexia nervosa and other neuropsychiatric conditions. The rationale for future research to investigate the combined use of low-dose ketamine and zinc supplementation to potentially extend the therapeutic benefits in anorexia nervosa is subsequently explored and promising biological markers for assessing and potentially predicting treatment response are outlined.
© 2023. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures
References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-5™. 5. Arlington: American Psychiatric Publishing, Inc.; 2013. p. xliv.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

