Hippocampal subfield viscoelasticity in amnestic mild cognitive impairment evaluated with MR elastography
- PMID: 36682312
- PMCID: PMC9871742
- DOI: 10.1016/j.nicl.2023.103327
Hippocampal subfield viscoelasticity in amnestic mild cognitive impairment evaluated with MR elastography
Abstract
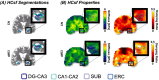
Hippocampal subfields (HCsf) are brain regions important for memory function that are vulnerable to decline with amnestic mild cognitive impairment (aMCI), which is often a preclinical stage of Alzheimer's disease. Studies in aMCI patients often assess HCsf tissue integrity using measures of volume, which has little specificity to microstructure and pathology. We use magnetic resonance elastography (MRE) to examine the viscoelastic mechanical properties of HCsf tissue, which is related to structural integrity, and sensitively detect differences in older adults with aMCI compared to an age-matched control group. Group comparisons revealed HCsf viscoelasticity is differentially affected in aMCI, with CA1-CA2 and DG-CA3 exhibiting lower stiffness and CA1-CA2 exhibiting higher damping ratio, both indicating poorer tissue integrity in aMCI. Including HCsf stiffness in a logistic regression improves classification of aMCI beyond measures of volume alone. Additionally, lower DG-CA3 stiffness predicted aMCI status regardless of DG-CA3 volume. These findings showcase the benefit of using MRE in detecting subtle pathological tissue changes in individuals with aMCI via the HCsf particularly affected in the disease.
Keywords: Brain; Hippocampus; Mechanical Properties; Mild Cognitive Impairment; Neurodegeneration; Stiffness.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., Gamst A., Holtzman D.M., Jagust W.J., Petersen R.C., Snyder P.J., Carrillo M.C., Thies B., Phelps C.H. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. - DOI - PMC - PubMed
-
- Benedict R.H.B., Schretlen D., Groninger L., Brandt J. Hopkins verbal learning test - Revised: Normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 1998;12:43–55. doi: 10.1076/clin.12.1.43.1726. - DOI
-
- Bigot M., Chauveau F., Amaz C., Sinkus R., Beuf O., Lambert S.A. The apparent mechanical effect of isolated amyloid-β and α-synuclein aggregates revealed by multi-frequency MRE. NMR Biomed. 2020;33:e4174. - PubMed
-
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

