Fasting and refeeding triggers specific changes in bile acid profiles and gut microbiota
- PMID: 36682739
- PMCID: PMC9934961
- DOI: 10.1111/1753-0407.13356
Fasting and refeeding triggers specific changes in bile acid profiles and gut microbiota
Abstract
Background: Bile acids (BAs) are closely related to nutrient supply and modified by gut microbiota. Gut microbiota perturbations shape BA composition, which further affects host metabolism.
Methods: We investigated BA profiles in plasma, feces, and liver of mice fed ad libitum, fasted for 24 h, fasted for 24 h and then refed for 24 h using ultraperformance liquid chromatography coupled to tandem mass spectrometry. Gut microbiota was measured by 16S rRNA gene sequencing. Expressions of BA biosynthesis-related genes in the liver and BA reabsorption-related genes in the ileum were analyzed.
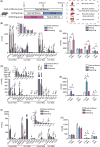
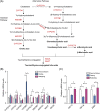
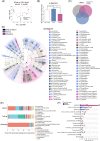
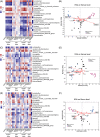
Findings: Compared with the controls, unconjugated primary BAs (PBAs) and unconjugated secondary BAs (SBAs) in plasma were decreased whereas conjugated SBAs in plasma, unconjugated PBAs, unconjugated SBAs and conjugated SBAs in feces, and unconjugated SBAs in liver were increased in the fasting mice. The expression of BA biosynthesis-related genes in the liver and BA reabsorption-related genes in the ileum were decreased in the fasting mice compared with the controls. Compared with the controls, Akkermansia, Parabacteroides, Muribaculum, Eubacterium_coprostanoligenes and Muribaculaceae were increased in the fasting mice whereas Lactobacillus and Bifidobacterium were decreased. All these changes in BAs and gut microbiota were recovered under refeeding. Akkermansia was negatively correlated with plasma levels of unconjugated PBAs, unconjugated SBAs and glucose, whereas it was positively correlated with plasma conjugated SBAs, fecal unconjugated PBAs, and fecal unconjugated SBAs.
Conclusions: We characterized the BA profiles, gut microbiota, and gene expression responsible for BA biosynthesis and intestinal reabsorption to explore their rapid changes in response to food availability. Our study highlighted the rapid effect of nutrient supply on BAs and gut microbiota.
背景:胆汁酸(BAs)与营养供应密切相关, 并受到肠道菌群的影响。肠道菌群改变BAs的组成, 进而影响宿主的代谢。 方法:采用超高效液相色谱-串联质谱(UPLC-MS/MS)检测小鼠自由进食、禁食24 h、禁食24 h后再进食24 h的血浆、粪便和肝脏中的BAs谱。采用16S rRNA基因测序检测肠道菌群, 分析肝脏中BA生物合成相关基因和回肠中BA重吸收相关基因的表达情况。 结果:与对照组相比, 空腹小鼠血浆中未偶联的初级BAs (PBAs)和未偶联的次级BAs (SBAs)减少, 而血浆中偶联的SBAs、粪便中未偶联的PBAs和SBAs以及肝脏中未偶联的SBAs增加。与对照组相比, 空腹小鼠肝脏中BA生物合成相关基因和回肠中BA再吸收相关基因表达降低。与对照组相比, 空腹小鼠中阿克曼菌属、狄氏副拟杆菌、肠鼠杆菌、产粪甾醇真杆菌和拟杆菌增加, 乳杆菌和双歧杆菌减少。这些改变在复食条件下得以恢复。阿克曼菌属与血浆未偶联PBAs、未偶联SBAs和葡萄糖呈负相关, 与血浆偶联SBAs、粪便未偶联PBAs和粪便未偶联SBAs呈正相关。 结论:本研究通过分析BA谱、肠道菌群和与BA合成和肠道重吸收相关的基因表达, 探索其在食物供应中的快速变化。我们的研究强调了营养供应对BAs和肠道菌群的快速影响。.
Keywords: bile acid; biosynthesis; fasting; gut microbiota; reabsorption; refeeding; 再吸收; 复食; 生物合成; 禁食; 肠道菌群; 胆汁酸.
© 2023 The Authors. Journal of Diabetes published by Ruijin Hospital, Shanghai JiaoTong University School of Medicine and John Wiley & Sons Australia, Ltd.
Figures
Similar articles
-
Circulating bile acids, bile acid receptor polymorphisms, and risk of chronic kidney disease among individuals with newly diagnosed type 2 diabetes: a prospective study.Am J Clin Nutr. 2025 Jul 25:S0002-9165(25)00433-2. doi: 10.1016/j.ajcnut.2025.07.017. Online ahead of print. Am J Clin Nutr. 2025. PMID: 40716521
-
Lactobacillus acidophilus ameliorates cholestatic liver injury through inhibiting bile acid synthesis and promoting bile acid excretion.Gut Microbes. 2024 Jan-Dec;16(1):2390176. doi: 10.1080/19490976.2024.2390176. Epub 2024 Aug 29. Gut Microbes. 2024. PMID: 39205654 Free PMC article. Clinical Trial.
-
Comprehensive Analysis of Serum and Fecal Bile Acid Profiles and Interaction with Gut Microbiota in Primary Biliary Cholangitis.Clin Rev Allergy Immunol. 2020 Feb;58(1):25-38. doi: 10.1007/s12016-019-08731-2. Clin Rev Allergy Immunol. 2020. PMID: 30900136
-
The molecular insights of bile acid homeostasis in host diseases.Life Sci. 2023 Oct 1;330:121919. doi: 10.1016/j.lfs.2023.121919. Epub 2023 Jul 6. Life Sci. 2023. PMID: 37422071 Review.
-
Bile acid metabolism and signaling: Emerging pharmacological targets of dietary polyphenols.Pharmacol Ther. 2023 Aug;248:108457. doi: 10.1016/j.pharmthera.2023.108457. Epub 2023 Jun 1. Pharmacol Ther. 2023. PMID: 37268113 Free PMC article. Review.
Cited by
-
Intermittent Fasting Improves Insulin Resistance by Modulating the Gut Microbiota and Bile Acid Metabolism in Diet-Induced Obesity.Mol Nutr Food Res. 2024 Nov;68(22):e2400451. doi: 10.1002/mnfr.202400451. Epub 2024 Nov 9. Mol Nutr Food Res. 2024. PMID: 39520336 Free PMC article.
-
Intermittent fasting and metabolic dysfunction-associated steatotic liver disease: the potential role of the gut-liver axis.Cell Biosci. 2025 May 23;15(1):64. doi: 10.1186/s13578-025-01406-w. Cell Biosci. 2025. PMID: 40410852 Free PMC article. Review.
-
Modeling "Two-Hit" Severe Pneumonia in Mice: Pathological Characteristics and Mechanistic Studies.Inflammation. 2025 Jun;48(3):1460-1483. doi: 10.1007/s10753-024-02136-w. Epub 2024 Aug 30. Inflammation. 2025. PMID: 39212889
-
Calorie restriction mimetic drugs could favorably influence gut microbiota leading to lifespan extension.Geroscience. 2023 Dec;45(6):3475-3490. doi: 10.1007/s11357-023-00851-0. Epub 2023 Jun 30. Geroscience. 2023. PMID: 37389698 Free PMC article. Review.
-
The gastrointestinal tract microbiome of Holstein × Angus cross cattle is negatively impacted by the pre-harvest process.Appl Environ Microbiol. 2025 May 21;91(5):e0259924. doi: 10.1128/aem.02599-24. Epub 2025 Apr 11. Appl Environ Microbiol. 2025. PMID: 40214226 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 82170819/National Natural Science Foundation of China
- 92157204/National Natural Science Foundation of China
- 81930021/National Natural Science Foundation of China
- 82250901/National Natural Science Foundation of China
- 81970691/National Natural Science Foundation of China
- 81900769/National Natural Science Foundation of China
- 82100916/National Natural Science Foundation of China
- 20224Y0087/Clinical Research Project of Shanghai Municipal Health Commission
- Innovative research team of high-level local universities in Shanghai
- 2021YFA1301100/National Key Research and Development Program of China
- 2021YFA1301103/National Key Research and Development Program of China
- 2022YFC2505202/National Key Research and Development Program of China
- SHDC2020CR1001A/Clinical Research Plan of SHDC
- SHDC2020CR3064B/Clinical Research Plan of SHDC
- 19411964200/Science and Technology Committee of Shanghai
- 20Y11905100/Science and Technology Committee of Shanghai
- DMRFP_I_01/Shanghai Medical and Health Development Foundation
- 20171903 Round 2/Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support
- 20XD1422800/Shanghai Outstanding Academic Leaders Plan
LinkOut - more resources
Full Text Sources

