Treatment outcomes of alternating chemoradiotherapy for nasopharyngeal carcinoma: a single-center safety and efficacy study
- PMID: 36682990
- PMCID: PMC10164767
- DOI: 10.1016/j.bjorl.2022.12.004
Treatment outcomes of alternating chemoradiotherapy for nasopharyngeal carcinoma: a single-center safety and efficacy study
Abstract
Objective: To evaluate the efficacy and safety of Alternating Chemoradiotherapy (ACRT) using cisplatin and 5-Fluorouracil (5-FU) in patients with nasopharyngeal carcinoma.
Methods: This was a retrospective study in which patients' clinical records were reviewed to identify patients with a new diagnosis of nasopharyngeal carcinoma at our institution between January 2005 and January 2019. Thirty-seven eligible patients were identified; of these, the clinical details of 27 patients treated with ACRT were evaluated. Patient outcomes, including overall survival and progression-free survival, and adverse events were assessed.
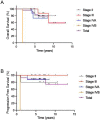
Results: Of these initial 37 patients, 1, 10, 13, 10, and 3 were staged as I, II, III, IVA, and IVB, respectively, as defined by the 8th edition of the TNM classification system. Twenty-seven patients received ACRT comprising sequential administration of chemotherapy, radiotherapy (wide field), chemotherapy, radiotherapy (shrinking field), and chemotherapy. The 5-year overall survival and progression-free survival rates were 83.7% and 88.9%, respectively. Treatment compliance was 93%, which is comparable to that of previous reports.
Conclusion: ACRT using cisplating and 5-fluorouracil was well tolerated with acceptable efficacy.
Level of evidence: IVa.
Keywords: 5-fluorouracil; Alternating chemoradiotherapy; Cisplatin; Nasopharyngeal carcinoma.
Copyright © 2022 Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial. Published by Elsevier España, S.L.U. All rights reserved.
Figures

References
-
- Chen Y.P., Tang L.L., Yang Q., Poh S.-S., Hui E.P., Chan A.T.C., et al. Induction chemotherapy plus concurrent chemoradiotherapy in endemic nasopharyngeal carcinoma: individual patient data pooled analysis of four randomized trials. Clin Cancer Res. 2018;24:1824–1833. - PubMed
-
- Colevas A.D., Yom S.S., Pfister D.G., Spencer S., Adelstein D., Adkins D., et al. NCCN guidelines insights: head and neck cancers, Version 1.2018. J Natl Compr Canc Netw. 2018;16:479–490. - PubMed
-
- Rodriguez-Galindo C., Krailo M.D., Krasin M.J., Huang L., McCarville M.B., Hicks J., et al. Treatment of childhood nasopharyngeal carcinoma with induction chemotherapy and concurrent chemoradiotherapy: results of the Children’s Oncology Group ARAR0331 Study. J Clin Oncol. 2019;37:3369–3376. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

