Manual for abdominal ultrasound in cancer screening and health checkups, revised edition (2021)
- PMID: 36683097
- PMCID: PMC9868003
- DOI: 10.1007/s10396-022-01272-w
Manual for abdominal ultrasound in cancer screening and health checkups, revised edition (2021)
Keywords: Abdominal ultrasonography; Cancer screening; Category; Heath checkup; Ultrasound finding; Ultrasound screening.
Conflict of interest statement
Members, etc., who created this manual were asked to declare the following conflicts of interest. With respect to companies/organizations from which the member himself or herself, a spouse, a first-degree or closer relative, an individual with whom he or she shares income/assets, or the director of an institution/division to which he or she belongs received some form of compensation: compensation (≥ 1 million yen), profit from stock (≥ 1 million yen, or ≥ 5%), patent royalty (≥ 1 million yen), lecture fee, etc. (≥ 500,000 yen), manuscript fee (≥ 500,000 yen), research expenses/grant (≥ 1 million yen), scholarship donation, etc. (≥ 1 million yen), endowed course provided by corporation, etc. (≥ 1 million yen), and provision of funds not directly related to research (≥ 50,000 yen). Masahiro OGAWA (compensation: GE Healthcare Japan, Canon Medical Systems, Dainippon Sumitomo Pharma), Shinji OKANIWA (compensation: GE Healthcare Japan, Canon Medical Systems), Masaki ADACHI (compensation: Medical Egg), Masayuki KITANO (lecture fee: Olympus, EA Pharma, Kaneka Medix, scholarship donation: AbbVie, Takeda Pharmaceutical), Masahiko NAKATA (scholarship donation: Hitachi Healthcare Business Unit), Takashi NISHIMURA(scholarship donation: Canon Medical Systems GE Healthcare Japan), Hiroshi MATSUO (lecture fee: Daiichi Sankyo).
Figures

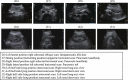

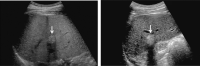
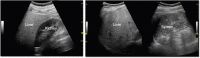

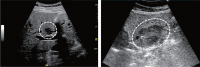
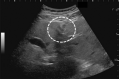
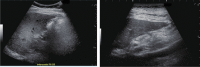
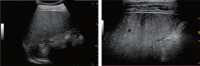
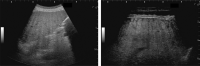
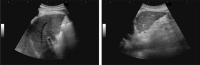
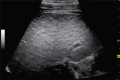
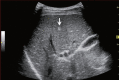
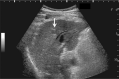
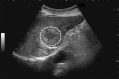
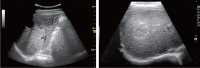
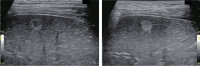
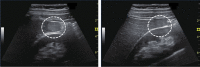

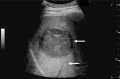
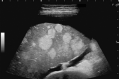

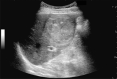
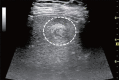
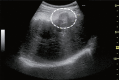

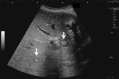
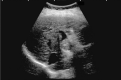
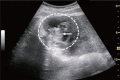
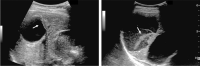

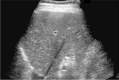
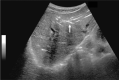
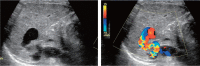


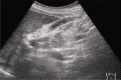
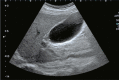
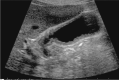

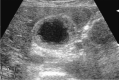
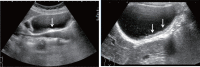
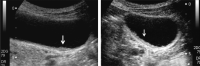
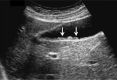

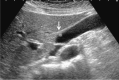
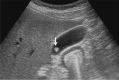
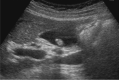

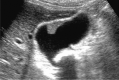
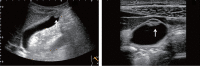
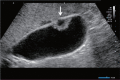
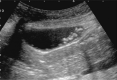
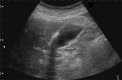
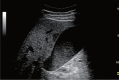



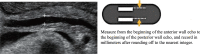
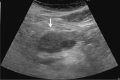
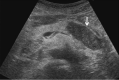
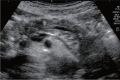
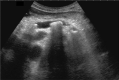
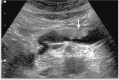
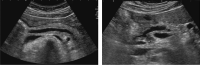
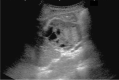
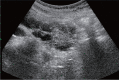
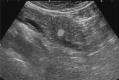

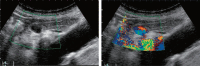
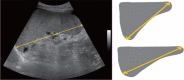
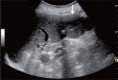
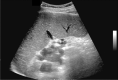
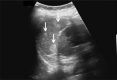
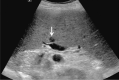

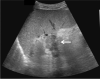
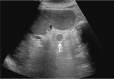
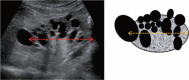

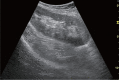
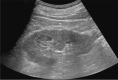
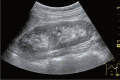
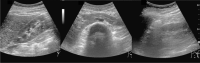



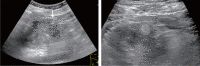
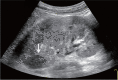
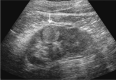
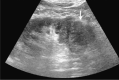
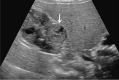
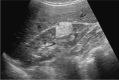
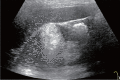
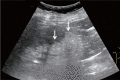
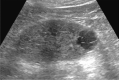
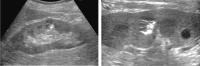
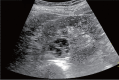

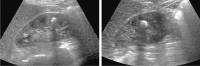
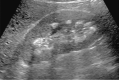

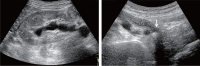
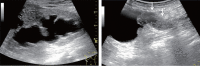
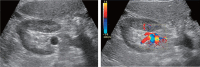
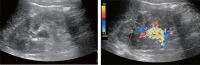
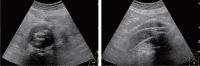
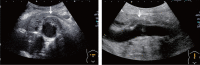
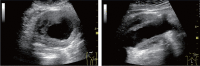
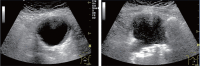
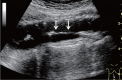
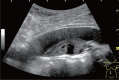
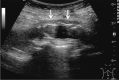
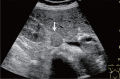
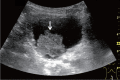
Comment in
-
Letter to the Editor: Ultrasound criteria for T1b or T2 gallbladder carcinoma.J Med Ultrason (2001). 2024 Jan;51(1):147-148. doi: 10.1007/s10396-023-01364-1. Epub 2023 Sep 23. J Med Ultrason (2001). 2024. PMID: 37740865 No abstract available.
References
-
- Working Group on Revision of the Manual for Abdominal Ultrasound in Cancer Screening and Health Checkups, Ultrasound Screening Committee of the Japanese Society of Gastrointestinal Cancer Screening et al. Manual for abdominal ultrasound in cancer screening and health checkups. J Gastrointest Cancer Screen. 2014;52:471–493.
-
- Committee of Abdominal Ultrasound Department for the Creation of Guidelines for Judgment of Imaging Examination by The Japan Society of Ningen Dock. Manual for abdominal ultrasound in cancer screening and health checkups. J Gastrointest Cancer Screen. 2015,http://www.ningen-dock.jp/wp/common/data/other/inspection/m_ultrasound_e.... (In Japanese, accessed on 5 Aug 2022).
-
- Subcommittee on the Manual for Abdominal Ultrasound in Cancer Screening and Health Checkups Manual for Abdominal Ultrasound in Cancer Screening and Health Checkups, Terminology and Diagnostic Criteria Committee of The Japan Society of Ultrasonics in Medicine. Jpn J Med Ultrasonics. 2015;42:201–24 (In Japanese).
-
- Working Group on Creation of the Manual for Abdominal Ultrasound in Cancer Screening and Health Checkups, Ultrasound Screening Committee of the Japanese Society of Gastrointestinal Cancer Screening Manual for Abdominal Ultrasound Cancer Screening. J Gastrointest Cancer Screen. 2011;49:549–565.
-
- Sachiko T, Shinji O, Takashi K, et al. Outline of the guideline for abdominal ultrasound cancer screening. Jpn J Med Ultrasonics. 2013;40:549–565. doi: 10.3179/jjmu.JJMU.R.7. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

