Gut Microbiome Associated With Graves Disease and Graves Orbitopathy: The INDIGO Multicenter European Study
- PMID: 36683389
- PMCID: PMC10807910
- DOI: 10.1210/clinem/dgad030
Gut Microbiome Associated With Graves Disease and Graves Orbitopathy: The INDIGO Multicenter European Study
Erratum in
-
Correction to "Gut Microbiome Associated With Graves Disease and Graves Orbitopathy: The INDIGO Multicenter European Study".J Clin Endocrinol Metab. 2024 Jan 18;109(2):e880. doi: 10.1210/clinem/dgad596. J Clin Endocrinol Metab. 2024. PMID: 37819778 Free PMC article. No abstract available.
Abstract
Context: Gut bacteria can influence host immune responses but little is known about their role in tolerance-loss mechanisms in Graves disease (GD; hyperthyroidism caused by autoantibodies, TRAb, to the thyrotropin receptor, TSHR) and its progression to Graves orbitopathy (GO).
Objective: This work aimed to compare the fecal microbiota in GD patients, with GO of varying severity, and healthy controls (HCs).
Methods: Patients were recruited from 4 European countries (105 GD patients, 41 HCs) for an observational study with cross-sectional and longitudinal components.
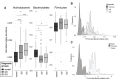
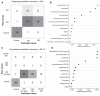
Results: At recruitment, when patients were hyperthyroid and TRAb positive, Actinobacteria were significantly increased and Bacteroidetes significantly decreased in GD/GO compared with HCs. The Firmicutes to Bacteroidetes (F:B) ratio was significantly higher in GD/GO than in HCs. Differential abundance of 15 genera was observed in patients, being most skewed in mild GO. Bacteroides displayed positive and negative correlations with TSH and free thyroxine, respectively, and was also significantly associated with smoking in GO; smoking is a risk factor for GO but not GD. Longitudinal analyses revealed that the presence of certain bacteria (Clostridiales) at diagnosis correlated with the persistence of TRAb more than 200 days after commencing antithyroid drug treatment.
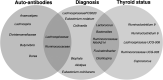
Conclusion: The increased F:B ratio observed in GD/GO mirrors our finding in a murine model comparing TSHR-immunized with control mice. We defined a microbiome signature and identified changes associated with autoimmunity as distinct from those due to hyperthyroidism. Persistence of TRAb is predictive of relapse; identification of these patients at diagnosis, via their microbiome, could improve management with potential to eradicate Clostridiales.
Keywords: Firmicutes:Bacteroidetes ratio; Graves disease; Graves orbitopathy; autoimmunity; gut microbiota; hyperthyroidism.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301‐316. - PubMed
-
- Prummel MF, Strieder T, Wiersinga WM. The environment and autoimmune thyroid diseases. Eur J Endocrinol. 2004;150(5):605‐618. - PubMed
-
- Köhling HL, Plummer SF, Marchesi JR, Davidge KS, Ludgate M. The microbiota and autoimmunity: their role in thyroid autoimmune diseases. Clin Immunol. 2017;183:63‐74. - PubMed
-
- Benvenga S, Guarneri F. Molecular mimicry and autoimmune thyroid disease. Rev Endocr Metab Disord. 2016;17(4):485‐498. - PubMed

