DELLA proteins regulate spore germination and reproductive development in Physcomitrium patens
- PMID: 36683399
- PMCID: PMC10952515
- DOI: 10.1111/nph.18756
DELLA proteins regulate spore germination and reproductive development in Physcomitrium patens
Abstract
Proteins of the DELLA family integrate environmental signals to regulate growth and development throughout the plant kingdom. Plants expressing non-degradable DELLA proteins underpinned the development of high-yielding 'Green Revolution' dwarf crop varieties in the 1960s. In vascular plants, DELLAs are regulated by gibberellins, diterpenoid plant hormones. How DELLA protein function has changed during land plant evolution is not fully understood. We have examined the function and interactions of DELLA proteins in the moss Physcomitrium (Physcomitrella) patens, in the sister group of vascular plants (Bryophytes). PpDELLAs do not undergo the same regulation as flowering plant DELLAs. PpDELLAs are not degraded by diterpenes, do not interact with GID1 gibberellin receptor proteins and do not participate in responses to abiotic stress. PpDELLAs do share a function with vascular plant DELLAs during reproductive development. PpDELLAs also regulate spore germination. PpDELLAs interact with moss-specific photoreceptors although a function for PpDELLAs in light responses was not detected. PpDELLAs likely act as 'hubs' for transcriptional regulation similarly to their homologues across the plant kingdom. Taken together, these data demonstrate that PpDELLA proteins share some biological functions with DELLAs in flowering plants, but other DELLA functions and regulation evolved independently in both plant lineages.
Keywords: Physcomitrium patens; DELLA proteins; diterpenes; light receptors; reproduction; spores; transcriptional regulation.
© 2023 The Authors. New Phytologist © 2023 New Phytologist Foundation.
Conflict of interest statement
None declared.
Figures



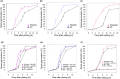


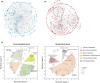

References
-
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. 2006. Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94. - PubMed
-
- Achard P, Renou JP, Berthome R, Harberd NP, Genschik P. 2008. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Current Biology 18: 656–660. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. - PubMed
-
- Aya K, Hiwatashi Y, Kojima M, Sakakibara H, Ueguchi‐Tanaka M, Hasebe M, Matsuoka M. 2011. The Gibberellin perception system evolved to regulate a pre‐existing GAMYB‐mediated system during land plant evolution. Nature Communications 2: 544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

