Deletion of Androgen Receptor in LepRb Cells Improves Estrous Cycles in Prenatally Androgenized Mice
- PMID: 36683455
- PMCID: PMC10091504
- DOI: 10.1210/endocr/bqad015
Deletion of Androgen Receptor in LepRb Cells Improves Estrous Cycles in Prenatally Androgenized Mice
Abstract
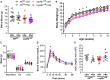
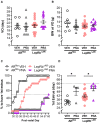
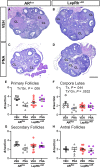
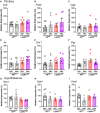
Androgens are steroid hormones crucial for sexual differentiation of the brain and reproductive function. In excess, however, androgens may decrease fertility as observed in polycystic ovary syndrome, a common endocrine disorder characterized by oligo/anovulation and/or polycystic ovaries. Hyperandrogenism may also disrupt energy homeostasis, inducing higher central adiposity, insulin resistance, and glucose intolerance, which may exacerbate reproductive dysfunction. Androgens bind to androgen receptors (ARs), which are expressed in many reproductive and metabolic tissues, including brain sites that regulate the hypothalamo-pituitary-gonadal axis and energy homeostasis. The neuronal populations affected by androgen excess, however, have not been defined. We and others have shown that, in mice, AR is highly expressed in leptin receptor (LepRb) neurons, particularly in the arcuate (ARH) and the ventral premammillary nuclei (PMv). Here, we assessed if LepRb neurons, which are critical in the central regulation of energy homeostasis and exert permissive actions on puberty and fertility, have a role in the pathogenesis of female hyperandrogenism. Prenatally androgenized (PNA) mice lacking AR in LepRb cells (LepRbΔAR) show no changes in body mass, body composition, glucose homeostasis, or sexual maturation. They do show, however, a remarkable improvement of estrous cycles combined with normalization of ovary morphology compared to PNA controls. Our findings indicate that the prenatal androgenization effects on adult reproductive physiology (ie, anestrus and anovulation) are mediated by a subpopulation of LepRb neurons directly sensitive to androgens. They also suggest that the effects of hyperandrogenism on sexual maturation and reproductive function in adult females are controlled by distinct neural circuits.
Keywords: androgen receptor; estrous cycle; hyperandrogenism; hypothalamus; leptin receptor.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Lack of AR in LepRb Cells Disrupts Ambulatory Activity and Neuroendocrine Axes in a Sex-Specific Manner in Mice.Endocrinology. 2020 Aug 1;161(8):bqaa110. doi: 10.1210/endocr/bqaa110. Endocrinology. 2020. PMID: 32609838 Free PMC article.
-
Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder.Proc Natl Acad Sci U S A. 2004 May 4;101(18):7129-34. doi: 10.1073/pnas.0308058101. Epub 2004 Apr 19. Proc Natl Acad Sci U S A. 2004. PMID: 15096602 Free PMC article.
-
AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS, AMERICAN COLLEGE OF ENDOCRINOLOGY, AND ANDROGEN EXCESS AND PCOS SOCIETY DISEASE STATE CLINICAL REVIEW: GUIDE TO THE BEST PRACTICES IN THE EVALUATION AND TREATMENT OF POLYCYSTIC OVARY SYNDROME--PART 1.Endocr Pract. 2015 Nov;21(11):1291-300. doi: 10.4158/EP15748.DSC. Endocr Pract. 2015. PMID: 26509855
-
Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies.J Clin Endocrinol Metab. 2006 May;91(5):1660-6. doi: 10.1210/jc.2005-2757. Epub 2006 Mar 7. J Clin Endocrinol Metab. 2006. PMID: 16522691 Review.
-
Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies.Mol Metab. 2020 May;35:100937. doi: 10.1016/j.molmet.2020.01.001. Epub 2020 Feb 5. Mol Metab. 2020. PMID: 32244180 Free PMC article.
Cited by
-
The ventral premammillary nucleus at the interface of environmental cues and social behaviors.Front Neurosci. 2025 Apr 10;19:1589156. doi: 10.3389/fnins.2025.1589156. eCollection 2025. Front Neurosci. 2025. PMID: 40276575 Free PMC article. Review.
-
Androgen excess: a hallmark of polycystic ovary syndrome.Front Endocrinol (Lausanne). 2023 Dec 13;14:1273542. doi: 10.3389/fendo.2023.1273542. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38152131 Free PMC article. Review.
-
Comparative Analysis of GnRH Pulse Generator Activity in Intact and Gonadectomized Male and Female Mice.Endocrinology. 2025 Jun 10;166(8):bqaf099. doi: 10.1210/endocr/bqaf099. Endocrinology. 2025. PMID: 40539639 Free PMC article.
-
Metabolomics and transcriptomics analyses reveal the complex molecular mechanisms by which the hypothalamus regulates sexual development in female goats.BMC Genomics. 2025 Mar 28;26(1):303. doi: 10.1186/s12864-025-11492-2. BMC Genomics. 2025. PMID: 40148778 Free PMC article.
References
-
- Azziz R, Carmina E, Chen Z, et al. . Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2(1):16057. - PubMed
-
- Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. - PubMed
-
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group . Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

