Roles of vaginal flora in human papillomavirus infection, virus persistence and clearance
- PMID: 36683675
- PMCID: PMC9848591
- DOI: 10.3389/fcimb.2022.1036869
Roles of vaginal flora in human papillomavirus infection, virus persistence and clearance
Abstract
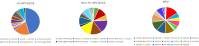
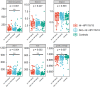
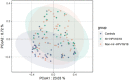
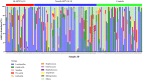
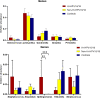
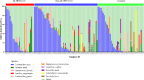
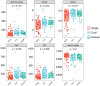
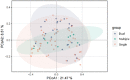
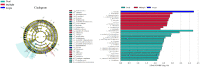
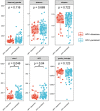
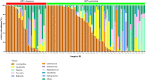
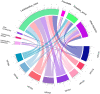
Vaginal flora plays a vital role in human papillomavirus (HPV) infection and progression to cancer. To reveal a role of the vaginal flora in HPV persistence and clearance, 90 patients with HPV infection and 45 healthy individuals were enrolled in this study and their vaginal flora were analyzed. Women with HPV infection were treated with Lactobacillus in the vaginal environment as a supplement to interferon therapy. Our results indicated that patients with high risk HPV (Hr-HPV) 16/18 infection had a significantly higher alpha diversity compared with the healthy control (p < 0.01), while there was no significant difference between the non-Hr-HPV16/18 group and the controls (p > 0.05). Patients with multiple HPV infection had insignificantly higher alpha diversity compared with single HPV infection (p > 0.05). The vaginal flora of patients with HPV infection exhibited different compositions when compared to the healthy controls. The dominant bacteria with the highest prevalence in HPV-positive group were Lactobacillus iners (n = 49, 54.44%), and the top 3 dominant bacteria in the HPV-persistent group were Lactobacillus iners (n = 34, 53.13%), Sneathia amnii (n = 9, 14.06%), and Lactobacillus delbrueckii (n = 3, 4.69%). Patients with HPV clearance had significantly lower alpha diversity, and the flora pattern was also different between groups displaying HPV clearance vs. persistence. The patients with persistent HPV infection had significantly higher levels of Bacteroidaceae, Erysipelotrichaceae, Helicobacteraceae, Neisseriaceae, Streptococcaceae (family level), and Fusobacterium, Bacteroides, Neisseria, and Helicobacter (genus level) than patients who had cleared HPV (p < 0.05).
Importance: Our study revealed differences in vaginal flora patterns are associated with HPV persistence and its clearance. Interferon plus probiotics can greatly improve virus clearance in some patients. Distinguishing bacterial features associated with HPV clearance in patients would be helpful for early intervention and reverse persistent infection.
Keywords: 16S ribosomal DNA sequencing; human papillomavirus; lactobacillus; probiotics; vaginal microbiota.
Copyright © 2023 Zeng, Li, Jiao, Cai, Yao, Xu, Huang, Zhang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Vaginal microbiota changes of persistent human papillomavirus infection after cervical conization.Front Cell Infect Microbiol. 2025 Apr 14;15:1544794. doi: 10.3389/fcimb.2025.1544794. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40297613 Free PMC article.
-
Human papillomavirus molecular prevalence in south China and the impact on vaginal microbiome of unvaccinated women.mSystems. 2024 Sep 17;9(9):e0073824. doi: 10.1128/msystems.00738-24. Epub 2024 Aug 9. mSystems. 2024. PMID: 39120153 Free PMC article.
-
Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort.BMC Infect Dis. 2020 Aug 26;20(1):629. doi: 10.1186/s12879-020-05324-9. BMC Infect Dis. 2020. PMID: 32842982 Free PMC article.
-
Vaginal microecology and its role in human papillomavirus infection and human papillomavirus associated cervical lesions.APMIS. 2024 Dec;132(12):928-947. doi: 10.1111/apm.13356. Epub 2023 Nov 8. APMIS. 2024. PMID: 37941500 Review.
-
The interplay between human papillomavirus and vaginal microbiota in cervical cancer development.Virol J. 2023 Apr 19;20(1):73. doi: 10.1186/s12985-023-02037-8. Virol J. 2023. PMID: 37076931 Free PMC article. Review.
Cited by
-
Cervical microbiota dysbiosis associated with high-risk Human Papillomavirus infection.PLoS One. 2024 Apr 26;19(4):e0302270. doi: 10.1371/journal.pone.0302270. eCollection 2024. PLoS One. 2024. PMID: 38669258 Free PMC article.
-
Human papillomavirus and cervical cancer in the microbial world: exploring the vaginal microecology.Front Cell Infect Microbiol. 2024 Jan 25;14:1325500. doi: 10.3389/fcimb.2024.1325500. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38333037 Free PMC article. Review.
-
Vaginal microbiota changes of persistent human papillomavirus infection after cervical conization.Front Cell Infect Microbiol. 2025 Apr 14;15:1544794. doi: 10.3389/fcimb.2025.1544794. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40297613 Free PMC article.
-
Medicinal Mushrooms, Probiotics and Combination of Natural Compounds in the Management of HPV: A Comparative Look at Viral Clearance and Lesion Resolution.Viruses. 2025 Jul 2;17(7):942. doi: 10.3390/v17070942. Viruses. 2025. PMID: 40733559 Free PMC article. Review.
-
Cervicovaginal microbiota: a promising direction for prevention and treatment in cervical cancer.Infect Agent Cancer. 2024 Apr 19;19(1):13. doi: 10.1186/s13027-024-00573-8. Infect Agent Cancer. 2024. PMID: 38641803 Free PMC article. Review.
References
-
- Audirac-Chalifour A., Torres-Poveda K., Bahena-Román M., Téllez-Sosa J., Martínez-Barnetche J., Cortina-Ceballos B., et al. . (2016). Cervical microbiome and cytokine profile at various stages of cervical cancer: A pilot study. PLos One 11 (4), e0153274. doi: 10.1371/journal.pone.0153274 - DOI - PMC - PubMed
-
- Borgdorff H., Gautam R., Armstrong S. D., Xia D., Ndayisaba G. F., van Teijlingen N. H., et al. . (2016). Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 9 (3), 621–633. doi: 10.1038/mi.2015.86 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

