Insights from targeting transferrin receptors to develop vaccines for pathogens of humans and food production animals
- PMID: 36683691
- PMCID: PMC9853020
- DOI: 10.3389/fcimb.2022.1083090
Insights from targeting transferrin receptors to develop vaccines for pathogens of humans and food production animals
Abstract
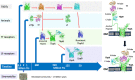
While developing vaccines targeting surface transferrin receptor proteins in Gram-negative pathogens of humans and food production animals, the common features derived from their evolutionary origins has provided us with insights on how improvements could be implemented in the various stages of research and vaccine development. These pathogens are adapted to live exclusively on the mucosal surfaces of the upper respiratory or genitourinary tract of their host and rely on their receptors to acquire iron from transferrin for survival, indicating that there likely are common mechanisms for delivering transferrin to the mucosal surfaces that should be explored. The modern-day receptors are derived from those present in bacteria that lived over 320 million years ago. The pathogens represent the most host adapted members of their bacterial lineages and may possess factors that enable them to have strong association with the mucosal epithelial cells, thus likely reside in a different niche than the commensal members of the bacterial lineage. The bacterial pathogens normally lead a commensal lifestyle which presents challenges for development of relevant infection models as most infection models either exclude the early stages of colonization or subsequent disease development, and the immune mechanisms at the mucosal surface that would prevent disease are not evident. Development of infection models emulating natural horizontal disease transmission are also lacking. Our aim is to share our insights from the study of pathogens of humans and food production animals with individuals involved in vaccine development, maintaining health or regulation of products in the human and animal health sectors.
Keywords: TBDT; evolution; host specifity; iron acquisition systems; microbioal community; transferrin; vaccine.
Copyright © 2023 Ewasechko, Chaudhuri and Schryvers.
Conflict of interest statement
AS is an inventor on several vaccine patents and is a co-owner of Engineered Antigens Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
How prevalent are lactoferrin receptors in Gram-negative bacteria?Biochem Cell Biol. 2025 Jan 1;103:1-12. doi: 10.1139/bcb-2024-0180. Epub 2025 Jan 9. Biochem Cell Biol. 2025. PMID: 39783447 Review.
-
Bacterial receptors for host transferrin and lactoferrin: molecular mechanisms and role in host-microbe interactions.Future Microbiol. 2013 Dec;8(12):1575-85. doi: 10.2217/fmb.13.125. Future Microbiol. 2013. PMID: 24266357 Review.
-
Are lactoferrin receptors in Gram-negative bacteria viable vaccine targets?Biometals. 2018 Jun;31(3):381-398. doi: 10.1007/s10534-018-0105-7. Epub 2018 May 16. Biometals. 2018. PMID: 29767396
-
Nonbinding site-directed mutants of transferrin binding protein B exhibit enhanced immunogenicity and protective capabilities.Infect Immun. 2015 Mar;83(3):1030-8. doi: 10.1128/IAI.02572-14. Epub 2014 Dec 29. Infect Immun. 2015. PMID: 25547790 Free PMC article.
-
Expression and functional analysis of Nile tilapia transferrin receptors (TfRs) in host resistance to pathogenic bacteria and iron ion metabolism.Fish Shellfish Immunol. 2020 May;100:407-417. doi: 10.1016/j.fsi.2020.03.027. Epub 2020 Mar 18. Fish Shellfish Immunol. 2020. PMID: 32200071
Cited by
-
Unveiling the immune dynamics of Neisseria persistent oral colonization.Infect Immun. 2024 Jul 11;92(7):e0004824. doi: 10.1128/iai.00048-24. Epub 2024 May 30. Infect Immun. 2024. PMID: 38814083 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

