Effects of Zhishi Daozhi Decoction on the intestinal flora of nonalcoholic fatty liver disease mice induced by a high-fat diet
- PMID: 36683694
- PMCID: PMC9846642
- DOI: 10.3389/fcimb.2022.1005318
Effects of Zhishi Daozhi Decoction on the intestinal flora of nonalcoholic fatty liver disease mice induced by a high-fat diet
Abstract
Background and aims: Nonalcoholic fatty liver disease (NAFLD) is the most common type of chronic liver disease with a high incidence, and the situation is not optimistic. Intestinal flora imbalance is strongly correlated with NAFLD pathogenesis. Zhishi Daozhi Decoction (ZDD) is a water decoction of the herbs used in the classical Chinese medicine prescription Zhishi Daozhi Pills. Zhishi Daozhi Pills has shown promising hepatoprotective and hypolipidemic properties, but its specific mechanism remains unclear.
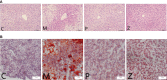
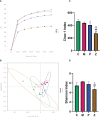
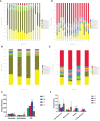
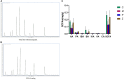
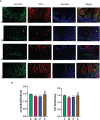
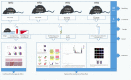
Methods: Mice were fed on a high fat-rich diet (HFD) for ten weeks, and then the animals were administrated ZDD through oral gavage for four weeks. The serum liver function and blood lipid indexes of the mice were then tested using an automatic biochemical analyzer. H&E and Oil Red O staining were used to observe the pathological conditions of mice liver tissue, and 16S rRNA sequencing technology was used to analyze the changes in intestinal flora of mice. The concentration of short-chain fatty acids (SCFAs) in the gut of mice was analyzed by gas chromatography-mass spectrometry (GC-MS). The expression of tight junction (TJ) proteins between ileal mucosal epithelial cells was analyzed using the immunofluorescence technique.
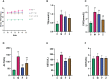
Results: ZDD was found to reduce the bodyweight of NAFLD mice, reduce serum TG, CHO, ALT, and AST levels, reduce fat accumulation in liver tissue, make the structure of intestinal flora comparable to the control group, and increase the concentration of intestinal SCFAs. It was also found to increase the expression of TJ proteins such as occludin and ZO-1, making them comparable to the control group.
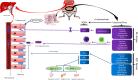
Conclusions: ZDD has a therapeutic effect on NAFLD mice induced by HFD, which may act by optimizing the intestinal flora structure.
Keywords: Zhishi Daozhi Decoction; high-fat diet; intestinal flora; nonalcoholic fatty liver disease; traditional Chinese medicine.
Copyright © 2023 Bi, Sun, Du, Chu, Li, Jia, Liu, Zhang, Li and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer GJ declared a shared affiliation with the authors CRB, JTS, JD, YJLi, XYJ, YL, WPZ, YJLiu to the handling editor at the time of review.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

