Therapeutic efficacy of dihydroartemisinin-piperaquine combination for the treatment of uncomplicated malaria in Ghana
- PMID: 36683700
- PMCID: PMC9853013
- DOI: 10.3389/fcimb.2022.1058660
Therapeutic efficacy of dihydroartemisinin-piperaquine combination for the treatment of uncomplicated malaria in Ghana
Abstract
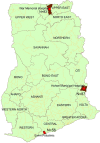
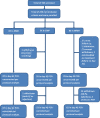
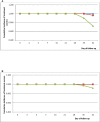
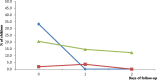
In 2020, Dihydroartemisinin-Piperaquine (DHAP) was adopted as a second-line antimalarial for treatment of uncomplicated malaria in Ghana following a review of the country's antimalarial medicines policy. Available data obtained in 2007 had shown PCR-uncorrected therapeutic efficacy of 93.3% using a 28-day follow-up schedule. In 2020, the standard 42-day follow-up schedule for DHAP was used to estimate efficacy levels among febrile children aged 6 months to 9 years in three malaria sentinel sites representing the three main ecological zones of the country- savannah, forest, and coastal. PCR genotyping distinguished between recrudescence and re-infection using merozoite surface protein 2 (MSP2)-specific primers for FC27 and 3D7 strains. Per protocol analyses showed day 28 efficacy of 100% in all three sentinel sites with day 42 PCR-corrected efficacy ranging between 90.3% (95% CI: 80.1 - 96.4%) in the savannah zone and 100% in the forest and coastal zones, yielding a national average of 97.0% (95% CI: 93.4 - 98.8). No day 3 parasitemia was observed in all three sites. Prevalence of measured fever (axillary temperature ≥ 37.5°C) declined from 50.0 - 98.8% on day 0 to 7.1-11.5% on day 1 whilst parasitemia declined from 100% on day 0 to 1.2 - 2.3% on day 1. Mean haemoglobin levels on days 28 and 42 were significantly higher than pre-treatment levels in all three sites. We conclude that DHAP is highly efficacious in the treatment of uncomplicated malaria in Ghana. This data will serve as baseline for subsequent DHAP efficacy studies in the country.
Keywords: Ghana; dihydroartemisinin-piperaquine; efficacy; treatment; uncomplicated malaria.
Copyright © 2023 Abuaku, Boateng, Peprah, Asamoah, Duah-Quashie, Matrevi, Amoako, Quashie, Owusu-Antwi, Malm and Koram.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Abuaku B., Duah-Quashie N. O., Quashie N., Gyasi A., Afriyie P. O., Owusu-Antwi F., et al. . (2021). Trends and predictive factors for treatment failure following artemisinin-based combination therapy among children with uncomplicated malaria in Ghana: 2005-2018. BMC Infect. Dis. 21 (1), 1255. doi: 10.1186/s12879-021-06961-4 - DOI - PMC - PubMed
-
- Abuaku B., Duah-Quashie N. O., Quaye L., Matrevi S. A., Quashie N., Gyasi A., et al. . (2019). Therapeutic efficacy of artesunate-amodiaquine and artemether-lumefantrine combinations for uncomplicated malaria in 10 sentinel sites across Ghana: 2015 – 2017. Malar. J. 18, 206. doi: 10.1186/s12936-019-2848-1 - DOI - PMC - PubMed
-
- Abuaku B., Duah N., Quaye L., Quashie N., Koram K. (2012). Therapeutic efficacy of artemether-lumefantrine combination in the treatment of uncomplicated malaria among children under five years of age in three ecological zones in Ghana. Malar. J. 11, 388. doi: 10.1186/1475-2875-11-388 - DOI - PMC - PubMed
-
- Abuaku B., Duah N., Quaye L., Quashie N., Malm K., Bart-Plange C., et al. . (2016). Therapeutic efficacy of artesunate-amodiaquine and artemether-lumefantrine combinations in the treatment of uncomplicated malaria in two ecological zones in Ghana. Malar. J. 15 (1), 6. doi: 10.1186/s12936-015-1080-x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

