Kinetics of the serological response up to one year after tularemia
- PMID: 36683705
- PMCID: PMC9853284
- DOI: 10.3389/fcimb.2022.1072703
Kinetics of the serological response up to one year after tularemia
Abstract
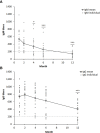
Serological analysis is the predominant method used to diagnose tularemia, a zoonotic disease caused by the highly virulent bacterium F. tularensis. We determined F. tularensis-specific IgM and IgG antibody titers by an LPS-based ELISA assay on five occasions one to twelve months after onset of ulceroglandular tularemia in 19 individuals. Peak IgM antibody titers were observed at the one-month time point and peak IgG antibody titers at the two-month time point. Both IgG and IgM antibody levels declined linearly thereafter with rather similar kinetics. Compared to the average one-month antibody titers, average IgG titers were not significantly lower before the 12-month time point and IgM titers before the 4-month time point. All, but one average titer, were significantly increased compared to the cut-off of the assay. Average IgG and IgM titers were significantly lower for the group = 69 years old compared to the group < 69 years. Collectively, the data demonstrate a persistence of F. tularensis-specific IgM and IgG antibody titers for at least 12 months after ulceroglandular tularemia. Thus, low, but significantly elevated F. tularensis-specific antibody titers are of limited diagnostic value since they are not indicative of ongoing tularemia.
Keywords: elderly; kinetics; one year; serological response; tularemia.
Copyright © 2023 Lindgren, Eklund, Eneslätt and Sjöstedt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Francisella tularensis-specific antibody levels in sera from Swedish patients with suspected tularemia during a 13-year period.Front Cell Infect Microbiol. 2024 Apr 2;14:1381776. doi: 10.3389/fcimb.2024.1381776. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38628552 Free PMC article.
-
Enzyme-linked immunosorbent assay for immunological diagnosis of human tularemia.J Clin Microbiol. 1979 Nov;10(5):615-21. doi: 10.1128/jcm.10.5.615-621.1979. J Clin Microbiol. 1979. PMID: 120873 Free PMC article.
-
Evaluation of In-House and Commercial Serological Tests for Diagnosis of Human Tularemia.J Clin Microbiol. 2017 Dec 26;56(1):e01440-17. doi: 10.1128/JCM.01440-17. Print 2018 Jan. J Clin Microbiol. 2017. PMID: 29118164 Free PMC article.
-
Francisella tularensis, Tularemia and Serological Diagnosis.Front Cell Infect Microbiol. 2020 Oct 26;10:512090. doi: 10.3389/fcimb.2020.512090. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194778 Free PMC article. Review.
-
[Francisella tularensis and tularemia in Turkey].Mikrobiyol Bul. 2007 Oct;41(4):621-36. Mikrobiyol Bul. 2007. PMID: 18173084 Review. Turkish.
Cited by
-
Targeting Tularemia: Clinical, Laboratory, and Treatment Outcomes From an 11-year Retrospective Observational Cohort in Northern Sweden.Clin Infect Dis. 2024 May 15;78(5):1222-1231. doi: 10.1093/cid/ciae098. Clin Infect Dis. 2024. PMID: 38393822 Free PMC article.
-
Francisella tularensis-specific antibody levels in sera from Swedish patients with suspected tularemia during a 13-year period.Front Cell Infect Microbiol. 2024 Apr 2;14:1381776. doi: 10.3389/fcimb.2024.1381776. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38628552 Free PMC article.
References
-
- Anda P., Pearson A., Tärnvik A. (2007). “Clinical expression in humans,” in WHO guidelines: Tularemia (World Health Organization, Geneva, Switzerland: WHO press; ), 11–19.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

