Specific fungi associated with response to capsulized fecal microbiota transplantation in patients with active ulcerative colitis
- PMID: 36683707
- PMCID: PMC9849685
- DOI: 10.3389/fcimb.2022.1086885
Specific fungi associated with response to capsulized fecal microbiota transplantation in patients with active ulcerative colitis
Abstract
Objective: Fecal microbiota transplantation (FMT) is a novel microbial treatment for patients with ulcerative colitis (UC). In this study, we performed a clinical trial of capsulized FMT in UC patients to determine the association between the gut fungal community and capsulized FMT outcomes.
Design: This study recruited patients with active UC (N = 22) and healthy individuals (donor, N = 9) according to the criteria. The patients received capsulized FMT three times a week. Patient stool samples were collected before (week 0) and after FMT follow-up visits at weeks 1, 4, and 12. Fungal communities were analysed using shotgun metagenomic sequencing.
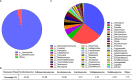
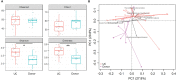
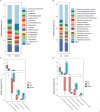
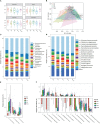
Results: According to metagenomic analysis, fungal community evenness index was greater in samples collected from patients, and the overall fungal community was clustered among the samples collected from donors. The dominant fungi in fecal samples collected from donors and patients were Ascomycota and Basidiomycota. However, capsulized FMT ameliorated microbial fungal diversity and altered fungal composition, based on metagenomic analysis of fecal samples collected before and during follow-up visits after capsulized FMT. Fungal diversity decreased in samples collected from patients who achieved remission after capsulized FMT, similar to samples collected from donors. Patients achieving remission after capsulized FMT had specific enrichment of Kazachstania naganishii, Pyricularia grisea, Lachancea thermotolerans, and Schizosaccharomyces pombe compared with patients who did not achieve remission. In addition, the relative abundance of P. grisea was higher in remission fecal samples during the follow-up visit. Meanwhile, decreased levels of pathobionts, such as Candida and Debaryomyces hansenii, were associated with remission in patients receiving capsulized FMT.
Conclusion: In the metagenomic analysis of fecal samples from donors and patients with UC receiving capsulized FMT, shifts in gut fungal diversity and composition were associated with capsulized FMT and validated in patients with active UC. We also identified the specific fungi associated with the induction of remission. ClinicalTrails.gov (NCT03426683).
Keywords: capsule administration; fecal microbiota transplantation; metagenomics; mycobiota; ulcerative colitis.
Copyright © 2023 Chen, Fan, Zhang, Yan, Chen, Wang, Hu, Huang, Su, Ren and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Capsulized Fecal Microbiota Transplantation Induces Remission in Patients with Ulcerative Colitis by Gut Microbial Colonization and Metabolite Regulation.Microbiol Spectr. 2023 Jun 15;11(3):e0415222. doi: 10.1128/spectrum.04152-22. Epub 2023 Apr 24. Microbiol Spectr. 2023. PMID: 37093057 Free PMC article. Clinical Trial.
-
Specific Bacteria and Metabolites Associated With Response to Fecal Microbiota Transplantation in Patients With Ulcerative Colitis.Gastroenterology. 2019 Apr;156(5):1440-1454.e2. doi: 10.1053/j.gastro.2018.12.001. Epub 2018 Dec 6. Gastroenterology. 2019. PMID: 30529583 Clinical Trial.
-
Long-term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: a prospective randomized pilot study.Microb Cell Fact. 2021 Jan 19;20(1):18. doi: 10.1186/s12934-021-01513-6. Microb Cell Fact. 2021. PMID: 33468164 Free PMC article. Clinical Trial.
-
Efficacy of Fecal Microbiota Transplantation in the Treatment of Active Ulcerative Colitis: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials.Inflamm Bowel Dis. 2023 May 2;29(5):808-817. doi: 10.1093/ibd/izac135. Inflamm Bowel Dis. 2023. PMID: 35766805
-
Fecal microbiota transplantation in ulcerative colitis.Acta Gastroenterol Belg. 2019 Oct-Dec;82(4):519-528. Acta Gastroenterol Belg. 2019. PMID: 31950808 Review.
Cited by
-
Current perspectives on fecal microbiota transplantation in inflammatory bowel disease.Indian J Gastroenterol. 2024 Feb;43(1):129-144. doi: 10.1007/s12664-023-01516-8. Epub 2024 Feb 9. Indian J Gastroenterol. 2024. PMID: 38334893 Review.
-
Research advancements and perspectives of inflammatory bowel disease: A comprehensive review.Sci Prog. 2024 Apr-Jun;107(2):368504241253709. doi: 10.1177/00368504241253709. Sci Prog. 2024. PMID: 38778725 Free PMC article. Review.
-
Unveiling the hidden players: exploring the role of gut mycobiome in cancer development and treatment dynamics.Gut Microbes. 2024 Jan-Dec;16(1):2328868. doi: 10.1080/19490976.2024.2328868. Epub 2024 Mar 14. Gut Microbes. 2024. PMID: 38485702 Free PMC article. Review.
-
The Role of Intestinal Fungi in the Pathogenesis and Treatment of Ulcerative Colitis.Microorganisms. 2025 Mar 31;13(4):794. doi: 10.3390/microorganisms13040794. Microorganisms. 2025. PMID: 40284630 Free PMC article. Review.
-
Exploring the role of gut microbiota in Parkinson's disease: insights from fecal microbiota transplantation.Front Neurosci. 2025 Jun 13;19:1574512. doi: 10.3389/fnins.2025.1574512. eCollection 2025. Front Neurosci. 2025. PMID: 40584885 Free PMC article. Review.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

