Locally controlled release of immunosuppressive promotes survival of transplanted adult spinal cord tissue
- PMID: 36683735
- PMCID: PMC9845520
- DOI: 10.1093/rb/rbac097
Locally controlled release of immunosuppressive promotes survival of transplanted adult spinal cord tissue
Abstract
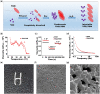
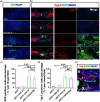
Transplantation of adult spinal cord tissue (aSCT) is a promising treatment for spinal cord injury (SCI) basing on various types of neural cells and matrix components inside aSCT. However, long-term systemic administration of immunosuppressors (e.g. tacrolimus, TAC) is required for the survival of allogeneic tissue, which often associated with severe side effects such as infection, liver damageand renal failure. In this study, a triglycerol monostearate (TGM)-based TAC delivery system (e.g. TAC@TGM) with high drug loading concentration was developed, which possessed injectable properties as well as sustainable and immune-responsive drug release behaviors. In complete transected SCI model, locally injected TAC@TGM could reduce the infiltration of inflammation cells, enhance the survival of transplanted aSCT (e.g. Tuj-1+ and NF+ neurons) and promote the recovery of locomotor function. Moreover, controlled release of TAC by TAC@TGM attenuated side effects of TAC on liver and kidneys compared with traditional systemic administration. More importantly, the developed TAC@TGM system provided a facile single dose of long-term immunosuppressive effect not just for aSCT transplantation, but also for other tissue/organ and cell transplantations.
Keywords: adult spinal cord tissue transplantation; controlled release; immune rejection; spinal cord injury; tacrolimus.
© The Author(s) 2022. Published by Oxford University Press.
Figures








References
-
- Liu D, Shen H, Shen Y, Long G, He X, Zhao Y, Yang Z, Dai J, Li X.. Dual-cues laden scaffold facilitates neurovascular regeneration and motor functional recovery after complete spinal cord injury. Adv Healthc Mater 2021;10:e2100089. - PubMed
-
- Sofroniew MV. Dissecting spinal cord regeneration. Nature 2018;557:343–50. - PubMed
-
- Reier PJ, Perlow MJ, Guth L.. Development of embryonic spinal cord transplants in the rat. Brain Res 1983;312:201–19. - PubMed
-
- Jakeman LB, Reier PJ.. Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J Comp Neurol 1991;307:311–34. - PubMed
LinkOut - more resources
Full Text Sources

