Transgenic PDGF-BB sericin hydrogel potentiates bone regeneration of BMP9-stimulated mesenchymal stem cells through a crosstalk of the Smad-STAT pathways
- PMID: 36683747
- PMCID: PMC9847547
- DOI: 10.1093/rb/rbac095
Transgenic PDGF-BB sericin hydrogel potentiates bone regeneration of BMP9-stimulated mesenchymal stem cells through a crosstalk of the Smad-STAT pathways
Abstract
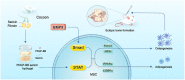
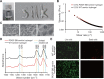
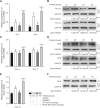
Silk as a natural biomaterial is considered as a promising bone substitute in tissue regeneration. Sericin and fibroin are the main components of silk and display unique features for their programmable mechanical properties, biocompatibility, biodegradability and morphological plasticity. It has been reported that sericin recombinant growth factors (GFs) can support cell proliferation and induce stem cell differentiation through cross-talk of signaling pathways during tissue regeneration. The transgenic technology allows the productions of bioactive heterologous GFs as fusion proteins with sericin, which are then fabricated into solid matrix or hydrogel format. Herein, using an injectable hydrogel derived from transgenic platelet-derived GF (PDGF)-BB silk sericin, we demonstrated that the PDGF-BB sericin hydrogel effectively augmented osteogenesis induced by bone morphogenetic protein (BMP9)-stimulated mesenchymal stem cells (MSCs) in vivo and in vitro, while inhibiting adipogenic differentiation. Further gene expression and protein-protein interactions studies demonstrated that BMP9 and PDGF-BB synergistically induced osteogenic differentiation through the cross-talk between Smad and Stat3 pathways in MSCs. Thus, our results provide a novel strategy to encapsulate osteogenic factors and osteoblastic progenitors in transgenic sericin-based hydrogel for robust bone tissue engineering.
Keywords: BMP9; biomaterials; growth factor; osteogenesis.
© The Author(s) 2022. Published by Oxford University Press.
Figures




Similar articles
-
Transgenic PDGF-BB/sericin hydrogel supports for cell proliferation and osteogenic differentiation.Biomater Sci. 2020 Jan 21;8(2):657-672. doi: 10.1039/c9bm01478k. Biomater Sci. 2020. PMID: 31769455
-
Leptin Potentiates BMP9-Induced Osteogenic Differentiation of Mesenchymal Stem Cells Through the Activation of JAK/STAT Signaling.Stem Cells Dev. 2020 Apr 15;29(8):498-510. doi: 10.1089/scd.2019.0292. Epub 2020 Mar 9. Stem Cells Dev. 2020. PMID: 32041483 Free PMC article.
-
Platelet-Rich Plasma Lysate-Incorporating Gelatin Hydrogel as a Scaffold for Bone Reconstruction.Bioengineering (Basel). 2022 Sep 29;9(10):513. doi: 10.3390/bioengineering9100513. Bioengineering (Basel). 2022. PMID: 36290482 Free PMC article.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
-
BMP9 is a potential therapeutic agent for use in oral and maxillofacial bone tissue engineering.Biochem Soc Trans. 2020 Jun 30;48(3):1269-1285. doi: 10.1042/BST20200376. Biochem Soc Trans. 2020. PMID: 32510140 Review.
Cited by
-
Sericin coats of silk fibres, a degumming waste or future material?Mater Today Bio. 2024 Oct 24;29:101306. doi: 10.1016/j.mtbio.2024.101306. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39534681 Free PMC article.
-
Smart Hydrogels for Bone Reconstruction via Modulating the Microenvironment.Research (Wash D C). 2023;6:0089. doi: 10.34133/research.0089. Epub 2023 Mar 27. Research (Wash D C). 2023. PMID: 36996343 Free PMC article. Review.
-
Application of stem cells in regeneration medicine.MedComm (2020). 2023 Jun 17;4(4):e291. doi: 10.1002/mco2.291. eCollection 2023 Aug. MedComm (2020). 2023. PMID: 37337579 Free PMC article. Review.
-
Biosynthesis of a dual growth factors (GFs) functionalized silk sericin hydrogel to promote chronic wound healing in diabetic mice.Bioact Mater. 2025 Jun 18;52:511-528. doi: 10.1016/j.bioactmat.2025.06.017. eCollection 2025 Oct. Bioact Mater. 2025. PMID: 40599340 Free PMC article.
-
Genetic Functionalization of Protein-Based Biomaterials via Protein Fusions.Biomacromolecules. 2024 Aug 12;25(8):4639-4662. doi: 10.1021/acs.biomac.4c00188. Epub 2024 Jul 29. Biomacromolecules. 2024. PMID: 39074364 Free PMC article. Review.
References
-
- Ahlfeld T, Schuster FP, Forster Y. et al. 3D plotted biphasic bone scaffolds for growth factor delivery: biological characterization in vitro and in vivo. Adv Healthc Mater 2019;8:e1801512. - PubMed
-
- Hsu EL, Stock SR.. Growth factors, carrier materials, and bone repair. Handb Exp Pharmacol 2020;262:121–56. - PubMed
-
- Midha S, Murab S, Ghosh S.. Osteogenic signaling on silk-based matrices. Biomaterials 2016;97:133–53. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

