Injectable and biofunctionalized fibrin hydrogels co-embedded with stem cells induce hair follicle genesis
- PMID: 36683749
- PMCID: PMC9847531
- DOI: 10.1093/rb/rbac086
Injectable and biofunctionalized fibrin hydrogels co-embedded with stem cells induce hair follicle genesis
Abstract
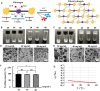
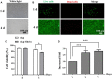
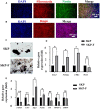
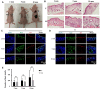
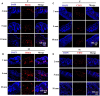
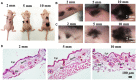
Fibrin-based hydrogels have been widely used in various tissue engineering because of their biocompatibility, biodegradability, tunable mechanical characteristics and nanofibrous structural properties. However, their ability to support stem cells for hair follicle neogenesis is unclear. In this study, we investigated the effect of fibrin hydrogels in supporting skin-derived precursors (SKPs) in hair follicle neogenesis. Our results showed that SKPs in fibrin hydrogels with high cell viability and proliferation, the stemness of SKPs could be maintained, and the expression of hair induction signature genes such as akp2 and nestin was enhanced. Moreover, hair follicle reconstruction experiments showed de novo hair genesis in mice and the hairs persisted for a long time without teratoma formation. More importantly, the blood vessels and sebaceous glands were also regenerated. Our study demonstrated that fibrin hydrogels are promising in hair follicle regeneration and have potential application in clinical settings for alopecia and wound healing.
Keywords: alopecia; fibrin hydrogels; fibrinogen; hair follicle neogenesis; skin-derived precursors.
© The Author(s) 2022. Published by Oxford University Press.
Figures

Similar articles
-
Self-assembling peptide hydrogel scaffolds support stem cell-based hair follicle regeneration.Nanomedicine. 2016 Oct;12(7):2115-2125. doi: 10.1016/j.nano.2016.05.021. Epub 2016 Jun 9. Nanomedicine. 2016. PMID: 27288668
-
Hair Follicle and Sebaceous Gland De Novo Regeneration With Cultured Epidermal Stem Cells and Skin-Derived Precursors.Stem Cells Transl Med. 2016 Dec;5(12):1695-1706. doi: 10.5966/sctm.2015-0397. Epub 2016 Jul 25. Stem Cells Transl Med. 2016. PMID: 27458264 Free PMC article.
-
Engineered Skin Substitute Regenerates the Skin with Hair Follicle Formation.Biomedicines. 2021 Apr 8;9(4):400. doi: 10.3390/biomedicines9040400. Biomedicines. 2021. PMID: 33917746 Free PMC article.
-
Wound Induced Hair Neogenesis - A Novel Paradigm for Studying Regeneration and Aging.Front Cell Dev Biol. 2020 Oct 15;8:582346. doi: 10.3389/fcell.2020.582346. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33178696 Free PMC article. Review.
-
A review of fibrin and fibrin composites for bone tissue engineering.Int J Nanomedicine. 2017 Jul 12;12:4937-4961. doi: 10.2147/IJN.S124671. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28761338 Free PMC article. Review.
Cited by
-
Material surface conjugated with fibroblast growth factor-2 for pluripotent stem cell culture and differentiation.Regen Biomater. 2025 Jan 2;12:rbaf003. doi: 10.1093/rb/rbaf003. eCollection 2025. Regen Biomater. 2025. PMID: 39967781 Free PMC article.
-
3D bioprinting of prefabricated artificial skin with multicomponent hydrogel for skin and hair follicle regeneration.Theranostics. 2025 Feb 10;15(7):2933-2950. doi: 10.7150/thno.104854. eCollection 2025. Theranostics. 2025. PMID: 40083946 Free PMC article.
-
Materials-based hair follicle engineering: Basic components and recent advances.Mater Today Bio. 2024 Oct 18;29:101303. doi: 10.1016/j.mtbio.2024.101303. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39498149 Free PMC article. Review.
-
Emerging biomedical engineering strategies for hair follicle regeneration.Bioact Mater. 2025 Jul 8;53:84-113. doi: 10.1016/j.bioactmat.2025.06.051. eCollection 2025 Nov. Bioact Mater. 2025. PMID: 40688016 Free PMC article. Review.
-
A double-network porous hydrogel based on high internal phase emulsions as a vehicle for potassium sucrose octasulfate delivery accelerates diabetic wound healing.Regen Biomater. 2024 Mar 12;11:rbae024. doi: 10.1093/rb/rbae024. eCollection 2024. Regen Biomater. 2024. PMID: 38628546 Free PMC article.
References
-
- Wang X, Tredget EE, Wu Y.. Dynamic signals for hair follicle development and regeneration. Stem Cells Dev 2012;21:7–18. - PubMed
-
- Hunt DP, Morris PN, Sterling J, Anderson JA, Joannides A, Jahoda C, Compston A, Chandran S.. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells 2008;26:163–72. - PubMed
-
- Schneider MR, Schmidt-Ullrich R, Paus R.. The hair follicle as a dynamic miniorgan. Curr Biol 2009;19:R132–42. - PubMed
-
- Hadshiew IM, Foitzik K, Arck PC, Paus R.. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol 2004;123:455–7. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

