NIR-II live imaging study on the degradation pattern of collagen in the mouse model
- PMID: 36683755
- PMCID: PMC9847529
- DOI: 10.1093/rb/rbac102
NIR-II live imaging study on the degradation pattern of collagen in the mouse model
Abstract
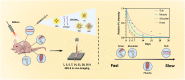
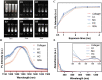
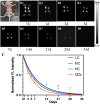
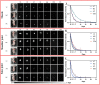
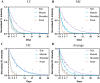
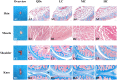
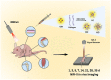
The degradation of collagen in different body parts is a critical point for designing collagen-based biomedical products. Here, three kinds of collagens labeled by second near-infrared (NIR-II) quantum dots (QDs), including collagen with low crosslinking degree (LC), middle crosslinking degree (MC) and high crosslinking degree (HC), were injected into the subcutaneous tissue, muscle and joints of the mouse model, respectively, in order to investigate the in vivo degradation pattern of collagen by NIR-II live imaging. The results of NIR-II imaging indicated that all tested collagens could be fully degraded after 35 days in the subcutaneous tissue, muscle and joints of the mouse model. However, the average degradation rate of subcutaneous tissue (k = 0.13) and muscle (k = 0.23) was slower than that of the joints (shoulder: k = 0.42, knee: k = 0.55). Specifically, the degradation rate of HC (k = 0.13) was slower than LC (k = 0.30) in muscle, while HC showed the fastest degradation rate in the shoulder and knee joints. In summary, NIR-II imaging could precisely identify the in vivo degradation rate of collagen. Moreover, the degradation rate of collagen was more closely related to the implanted body parts rather than the crosslinking degree of collagen, which was slower in the subcutaneous tissue and muscle compared to the joints in the mouse model.
Keywords: NIR-II live imaging; collagen; crosslinking degree; degradation rate; in vivo.
© The Author(s) 2022. Published by Oxford University Press.
Figures

References
-
- Shekhter AB, Fayzullin AL, Vukolova MN, Rudenko TG, Osipycheva VD, Litvitsky PF.. Medical applications of collagen and collagen-based materials. Curr Med Chem 2019;26:506–16. - PubMed
-
- An B, Lin YS, Brodsky B.. Collagen interactions: drug design and delivery. Adv Drug Deliv Rev 2016;97:69–84. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

