Fabrication of initial trabecular bone-inspired three-dimensional structure with cell membrane nano fragments
- PMID: 36683756
- PMCID: PMC9845518
- DOI: 10.1093/rb/rbac088
Fabrication of initial trabecular bone-inspired three-dimensional structure with cell membrane nano fragments
Abstract
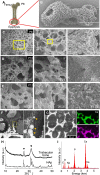
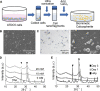
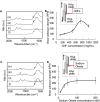
The extracellular matrix of trabecular bone has a large surface exposed to the bone marrow and plays important roles such as hematopoietic stem cell niche formation and maintenance. In vitro reproduction of trabecular bone microenvironment would be valuable not only for developing a functional scaffold for bone marrow tissue engineering but also for understanding its biological functions. Herein, we analyzed and reproduced the initial stages of trabecular bone formation in mouse femur epiphysis. We identified that the trabecular bone formation progressed through the following steps: (i) partial rupture of hypertrophic chondrocytes; (ii) calcospherite formation on cell membrane nano fragments (CNFs) derived from the ruptured cells; and (iii) calcospherite growth and fusion to form the initial three-dimensional (3D) structure of trabecular bones. For reproducing the initial trabecular bone formation in vitro, we collected CNFs from cultured cells and used as nucleation sites for biomimetic calcospherite formation. Strikingly, almost the same 3D structure of the initial trabecular bone could be obtained in vitro by using additional CNFs as a binder to fuse biomimetic calcospherites.
Keywords: bone tissue synthesis; calcospherites; cell membrane nano fragments; three dimensionalization; trabecular bone.
© The Author(s) 2022. Published by Oxford University Press.
Figures


Similar articles
-
Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells.Biomaterials. 2014 Jan;35(3):929-40. doi: 10.1016/j.biomaterials.2013.10.038. Epub 2013 Oct 28. Biomaterials. 2014. PMID: 24176196
-
[Treatment of a bone bridge by transplantation of mesenchymal stem cells and chondrocytes in a composite scaffold in pigs: experimental study].Acta Chir Orthop Traumatol Cech. 2011;78(6):528-36. Acta Chir Orthop Traumatol Cech. 2011. PMID: 22217406 Czech.
-
Polyurethane foam scaffold as in vitro model for breast cancer bone metastasis.Acta Biomater. 2017 Nov;63:306-316. doi: 10.1016/j.actbio.2017.09.017. Epub 2017 Sep 18. Acta Biomater. 2017. PMID: 28927931
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
-
Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering.Adv Healthc Mater. 2017 Dec;6(23). doi: 10.1002/adhm.201700612. Epub 2017 Nov 24. Adv Healthc Mater. 2017. PMID: 29171714 Review.
References
-
- Robling AG, Castillo AB, Turner CH.. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 2006;8:455–98. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

