Comparative Assessment of the Binding and Neutralisation Activity of Bispecific Antibodies Against SARS-CoV-2 Variants
- PMID: 36683765
- PMCID: PMC9847335
- DOI: 10.1093/abt/tbac032
Comparative Assessment of the Binding and Neutralisation Activity of Bispecific Antibodies Against SARS-CoV-2 Variants
Abstract
Background: Neutralising antibodies against SARS-CoV-2 are a vital component in the fight against COVID-19 pandemic, having the potential of both therapeutic and prophylactic applications. Bispecific antibodies (BsAbs) against SARS-CoV-2 are particularly promising, given their ability to bind simultaneously to two distinct sites of the receptor-binding domain (RBD) of the viral spike protein. Such antibodies are complex molecules associated with multi-faceted mechanisms of action that require appropriate bioassays to ensure product quality and manufacturing consistency.
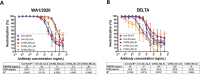
Methods: We developed procedures for biolayer interferometry (BLI) and a cell-based virus neutralisation assay, the focus reduction neutralisation test (FRNT). Using both assays, we tested a panel of five BsAbs against different spike variants (Ancestral, Delta and Omicron) to evaluate the use of these analytical methods in assessing binding and neutralisation activities of anti-SARS-CoV-2 therapeutics.
Results: We found comparable trends between BLI-derived binding affinity and FRNT-based virus neutralisation activity. Antibodies that displayed high binding affinity against a variant were often followed by potent neutralisation at lower concentrations, whereas those with low binding affinity also demonstrated reduced neutralisation activity.
Conclusion: The results support the utility of BLI and FRNT assays in measuring variant-specific binding and virus neutralisation activity of anti-SARS-CoV-2 antibodies.
Keywords: COVID-19; SARS-CoV-2; bioassay; bispecific antibody; product quality.
© The Author(s) 2022. Published by Oxford University Press on behalf of Antibody Therapeutics. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Sub-optimal neutralisation of omicron (B.1.1.529) variant by antibodies induced by vaccine alone or SARS-CoV-2 Infection plus vaccine (hybrid immunity) post 6-months.EBioMedicine. 2022 Apr;78:103938. doi: 10.1016/j.ebiom.2022.103938. Epub 2022 Mar 16. EBioMedicine. 2022. PMID: 35305396 Free PMC article.
-
A Bispecific Antibody Targeting RBD and S2 Potently Neutralizes SARS-CoV-2 Omicron and Other Variants of Concern.J Virol. 2022 Aug 24;96(16):e0077522. doi: 10.1128/jvi.00775-22. Epub 2022 Aug 2. J Virol. 2022. PMID: 35916510 Free PMC article.
-
Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT).Emerg Microbes Infect. 2020 Dec;9(1):2394-2403. doi: 10.1080/22221751.2020.1835448. Emerg Microbes Infect. 2020. PMID: 33043818 Free PMC article.
-
Passive Immunotherapy Against SARS-CoV-2: From Plasma-Based Therapy to Single Potent Antibodies in the Race to Stay Ahead of the Variants.BioDrugs. 2022 May;36(3):231-323. doi: 10.1007/s40259-022-00529-7. Epub 2022 Apr 27. BioDrugs. 2022. PMID: 35476216 Free PMC article. Review.
-
Susceptibility of SARS-CoV-2 Omicron Variants to Therapeutic Monoclonal Antibodies: Systematic Review and Meta-analysis.Microbiol Spectr. 2022 Aug 31;10(4):e0092622. doi: 10.1128/spectrum.00926-22. Epub 2022 Jun 14. Microbiol Spectr. 2022. PMID: 35700134 Free PMC article.
Cited by
-
Function and mechanism of bispecific antibodies targeting SARS-CoV-2.Cell Insight. 2024 Feb 3;3(2):100150. doi: 10.1016/j.cellin.2024.100150. eCollection 2024 Apr. Cell Insight. 2024. PMID: 38374826 Free PMC article. Review.
-
Fc gamma receptor polymorphisms in antibody therapy: implications for bioassay development to enhance product quality.Antib Ther. 2025 Jan 21;8(2):87-98. doi: 10.1093/abt/tbaf003. eCollection 2025 Apr. Antib Ther. 2025. PMID: 40177643 Review.
References
-
- US Food and Drug Administration . Emergency Use Authorization. 2020; 2020. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regula....
-
- Centers for Disease Control and Prevention (CDC), National Center for Immunization and Respiratory Diseases (NCRID), Division for Viral Diseases . SARS-CoV-2 Variant Classifications and Definitions. 2020; 2020. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classificatio....
LinkOut - more resources
Full Text Sources
Miscellaneous
