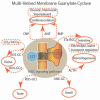
Multilimbed membrane guanylate cyclase signaling system, evolutionary ladder
- PMID: 36683846
- PMCID: PMC9849996
- DOI: 10.3389/fnmol.2022.1022771
Multilimbed membrane guanylate cyclase signaling system, evolutionary ladder
Abstract
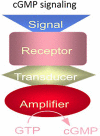
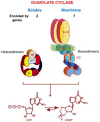
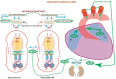
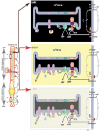
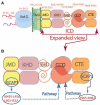
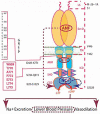
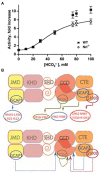
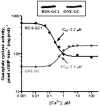
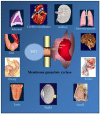
One monumental discovery in the field of cell biology is the establishment of the membrane guanylate cyclase signal transduction system. Decoding its fundamental, molecular, biochemical, and genetic features revolutionized the processes of developing therapies for diseases of endocrinology, cardio-vasculature, and sensory neurons; lastly, it has started to leave its imprints with the atmospheric carbon dioxide. The membrane guanylate cyclase does so via its multi-limbed structure. The inter-netted limbs throughout the central, sympathetic, and parasympathetic systems perform these functions. They generate their common second messenger, cyclic GMP to affect the physiology. This review describes an historical account of their sequential evolutionary development, their structural components and their mechanisms of interaction. The foundational principles were laid down by the discovery of its first limb, the ACTH modulated signaling pathway (the companion monograph). It challenged two general existing dogmas at the time. First, there was the question of the existence of a membrane guanylate cyclase independent from a soluble form that was heme-regulated. Second, the sole known cyclic AMP three-component-transduction system was modulated by GTP-binding proteins, so there was the question of whether a one-component transduction system could exclusively modulate cyclic GMP in response to the polypeptide hormone, ACTH. The present review moves past the first question and narrates the evolution and complexity of the cyclic GMP signaling pathway. Besides ACTH, there are at least five additional limbs. Each embodies a unique modular design to perform a specific physiological function; exemplified by ATP binding and phosphorylation, Ca2+-sensor proteins that either increase or decrease cyclic GMP synthesis, co-expression of antithetical Ca2+ sensors, GCAP1 and S100B, and modulation by atmospheric carbon dioxide and temperature. The complexity provided by these various manners of operation enables membrane guanylate cyclase to conduct diverse functions, exemplified by the control over cardiovasculature, sensory neurons and, endocrine systems.
Keywords: calcium; carbon dioxide; cardiovascular; cyclic GMP signaling pathways; membrane guanylate cyclase; sensory neurons; surface receptors; transduction modes.
Copyright © 2023 Duda and Sharma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Integrative Signaling Networks of Membrane Guanylate Cyclases: Biochemistry and Physiology.Front Mol Neurosci. 2016 Sep 15;9:83. doi: 10.3389/fnmol.2016.00083. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27695398 Free PMC article. Review.
-
Membrane guanylate cyclase, a multimodal transduction machine: history, present, and future directions.Front Mol Neurosci. 2014 Jul 2;7:56. doi: 10.3389/fnmol.2014.00056. eCollection 2014. Front Mol Neurosci. 2014. PMID: 25071437 Free PMC article. Review.
-
Antithetical modes of and the Ca(2+) sensors targeting in ANF-RGC and ROS-GC1 membrane guanylate cyclases.Front Mol Neurosci. 2012 Apr 9;5:44. doi: 10.3389/fnmol.2012.00044. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22509151 Free PMC article.
-
Differential Ca(2+) sensor guanylate cyclase activating protein modes of photoreceptor rod outer segment membrane guanylate cyclase signaling.Biochemistry. 2012 Jun 12;51(23):4650-7. doi: 10.1021/bi300572w. Epub 2012 Jun 1. Biochemistry. 2012. PMID: 22642846 Free PMC article.
-
Bicarbonate and Ca(2+) Sensing Modulators Activate Photoreceptor ROS-GC1 Synergistically.Front Mol Neurosci. 2016 Jan 28;9:5. doi: 10.3389/fnmol.2016.00005. eCollection 2016. Front Mol Neurosci. 2016. PMID: 26858600 Free PMC article.
Cited by
-
Homocysteine Attack on Vascular Endothelium-Old and New Features.Int J Mol Sci. 2025 Jun 30;26(13):6298. doi: 10.3390/ijms26136298. Int J Mol Sci. 2025. PMID: 40650078 Free PMC article. Review.
References
-
- Ahrens H., Paul A. K., Kuroda Y., Sharma R. K. (1982). Adrenocortical cyclic GMP-dependent protein kinase: purification, characterization, and modification of its activity by Calmodulin and its relationship with steroidogeneses. Arch. Biochem. Biophys. 215, 597–609. doi: 10.1016/0003-9861(82)90121-7, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

