Ex vivo analysis platforms for monitoring amyloid precursor protein cleavage
- PMID: 36683852
- PMCID: PMC9852844
- DOI: 10.3389/fnmol.2022.1068990
Ex vivo analysis platforms for monitoring amyloid precursor protein cleavage
Abstract
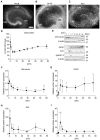
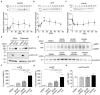
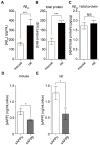
Alzheimer's disease (AD) is a progressive neurodegenerative brain disorder and the most common cause of dementia in the elderly. The presence of large numbers of senile plaques, neurofibrillary tangles, and cerebral atrophy is the characteristic feature of AD. Amyloid β peptide (Aβ), derived from the amyloid precursor protein (APP), is the main component of senile plaques. AD has been extensively studied using methods involving cell lines, primary cultures of neural cells, and animal models; however, discrepancies have been observed between these methods. Dissociated cultures lose the brain's tissue architecture, including neural circuits, glial cells, and extracellular matrix. Experiments with animal models are lengthy and require laborious monitoring of multiple parameters. Therefore, it is necessary to combine these experimental models to understand the pathology of AD. An experimental platform amenable to continuous observation and experimental manipulation is required to analyze long-term neuronal development, plasticity, and progressive neurodegenerative diseases. In the current study, we provide a practical method to slice and cultivate rodent hippocampus to investigate the cleavage of APP and secretion of Aβ in an ex vivo model. Furthermore, we provide basic information on Aβ secretion using slice cultures. Using our optimized method, dozens to hundreds of long-term stable slice cultures can be coordinated simultaneously. Our findings are valuable for analyses of AD mouse models and senile plaque formation culture models.
Keywords: Alzheimer’s disease; amyloid β; hippocampus; neurodegenerative disease; organotypic brain culture; secretase.
Copyright © 2023 Kamikubo, Jin, Zhou, Niisato, Hashimoto, Takasugi and Sakurai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
[Alzheimer disease: cellular and molecular aspects].Bull Mem Acad R Med Belg. 2005;160(10-12):445-9; discussion 450-1. Bull Mem Acad R Med Belg. 2005. PMID: 16768248 French.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Beta-amyloid, neuronal death and Alzheimer's disease.Curr Mol Med. 2001 Dec;1(6):733-7. doi: 10.2174/1566524013363177. Curr Mol Med. 2001. PMID: 11899259 Review.
-
Molecular and functional signatures in a novel Alzheimer's disease mouse model assessed by quantitative proteomics.Mol Neurodegener. 2018 Jan 16;13(1):2. doi: 10.1186/s13024-017-0234-4. Mol Neurodegener. 2018. PMID: 29338754 Free PMC article.
-
Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
Cited by
-
Early Age- and Sex-Dependent Regulation of Astrocyte-Mediated Glutamatergic Synapse Elimination in the Rat Prefrontal Cortex: Establishing an Organotypic Brain Slice Culture Investigating Tool.Cells. 2023 Dec 4;12(23):2761. doi: 10.3390/cells12232761. Cells. 2023. PMID: 38067189 Free PMC article.
-
Preclinical translational platform of neuroinflammatory disease biology relevant to neurodegenerative disease.J Neuroinflammation. 2024 Jan 31;21(1):37. doi: 10.1186/s12974-024-03029-3. J Neuroinflammation. 2024. PMID: 38297405 Free PMC article.
References
-
- Benjannet S., Elagoz A., Wickham L., Mamarbachi M., Munzer J. S., Basak A., et al. . (2001). Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro-and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J. Biol. Chem. 276, 10879–10887. doi: 10.1074/jbc.M009899200, PMID: - DOI - PubMed
-
- Bergold P. J., Casaccia-Bonnefil P., Zeng X. L., Federoff H. J. (1993). Transsynaptic neuronal loss induced in hippocampal slice cultures by a herpes simplex virus vector expressing the GluR6 subunit of the kainate receptor. Proc. Natl. Acad. Sci. U. S. A. 90, 6165–6169. doi: 10.1073/pnas.90.13.6165 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

