Nanoalbumin-prodrug conjugates prepared via a thiolation-and-conjugation method improve cancer chemotherapy and immune checkpoint blockade therapy by promoting CD8+ T-cell infiltration
- PMID: 36684090
- PMCID: PMC9842047
- DOI: 10.1002/btm2.10377
Nanoalbumin-prodrug conjugates prepared via a thiolation-and-conjugation method improve cancer chemotherapy and immune checkpoint blockade therapy by promoting CD8+ T-cell infiltration
Abstract
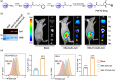
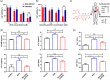
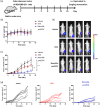
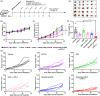
Protein-drug conjugates are emerging tools to combat cancers. Here, we adopted an indirect thiolation-and-conjugation method as a general strategy to prepare protein-drug conjugates. We found for the first time that this method led to the formation of nanometric conjugates, probably due to the formation of intermolecular disulfide bonds, which facilitated enhanced uptake by cancer cells. As a proof-of-concept application in cancer therapy, a nanometric albumin-doxorubicin prodrug conjugate (NanoAlb-proDOX) was prepared. The nanometric size promoted its uptake by cancer cells, and the prodrug characteristic defined its selective cytotoxicity toward cancer cells in vitro and reduced side effects in vivo. In multiple tumor xenograft models, nanometric NanoAlb-proDOX showed superior antitumor activity and synergy with immune checkpoint blockade, probably due to the synergistically enhanced tumor CD8+ T-cell infiltration and activation. Hence, the thiolation-and-conjugation strategy may serve as a generally applicable method for preparing drug conjugates, and the proof-of-concept nanometric albumin-doxorubicin conjugate may be a good choice for antitumor therapy with the ability to co-stimulate the efficacy of immune checkpoint blockade.
Keywords: CD8+ T cells; NanoAlb‐proDOX; immune checkpoint blockade; protein–drug conjugates; thiolation‐and‐conjugation.
© 2022 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Visible-Light-Triggered Prodrug Nanoparticles Combine Chemotherapy and Photodynamic Therapy to Potentiate Checkpoint Blockade Cancer Immunotherapy.ACS Nano. 2021 Jul 27;15(7):12086-12098. doi: 10.1021/acsnano.1c03416. Epub 2021 Jun 24. ACS Nano. 2021. PMID: 34165970
-
Dual functional immunostimulatory polymeric prodrug carrier with pendent indoximod for enhanced cancer immunochemotherapy.Acta Biomater. 2019 May;90:300-313. doi: 10.1016/j.actbio.2019.03.048. Epub 2019 Mar 28. Acta Biomater. 2019. PMID: 30930305 Free PMC article.
-
Tumor-specific delivery of doxorubicin through conjugation of pH-responsive peptide for overcoming drug resistance in cancer.Int J Pharm. 2017 Aug 7;528(1-2):322-333. doi: 10.1016/j.ijpharm.2017.06.022. Epub 2017 Jun 9. Int J Pharm. 2017. PMID: 28606508
-
Combining antibody-drug conjugates and immune-mediated cancer therapy: What to expect?Biochem Pharmacol. 2016 Feb 15;102:1-6. doi: 10.1016/j.bcp.2015.12.008. Epub 2015 Dec 11. Biochem Pharmacol. 2016. PMID: 26686577 Review.
-
Targeted cancer therapy: conferring specificity to cytotoxic drugs.Acc Chem Res. 2008 Jan;41(1):98-107. doi: 10.1021/ar700108g. Epub 2007 Aug 18. Acc Chem Res. 2008. PMID: 17705444 Review.
Cited by
-
Rationalized landscape on protein-based cancer nanomedicine: Recent progress and challenges.Int J Pharm X. 2024 Mar 11;7:100238. doi: 10.1016/j.ijpx.2024.100238. eCollection 2024 Jun. Int J Pharm X. 2024. PMID: 38511068 Free PMC article. Review.
-
Challenges in Preparation of Albumin Nanoparticle-Based Radiopharmaceuticals.Molecules. 2022 Dec 6;27(23):8596. doi: 10.3390/molecules27238596. Molecules. 2022. PMID: 36500689 Free PMC article. Review.
-
The application of tissue engineering strategies for uterine regeneration.Mater Today Bio. 2025 Feb 18;31:101594. doi: 10.1016/j.mtbio.2025.101594. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40070871 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. - PubMed
-
- Eifel PJ. Concurrent chemotherapy and radiation therapy as the standard of care for cervical cancer. Nat Clin Pract Oncol. 2006;3(5):248‐255. - PubMed
-
- Mazhar D, Waxman J. Early chemotherapy in prostate cancer. Nat Clin Pract Urol. 2008;5(9):486‐493. - PubMed
-
- Kroon J, Kooijman S, Cho NJ, Storm G, van der Pluijm G. Improving taxane‐based chemotherapy in castration‐resistant prostate cancer. Trends Pharmacol Sci. 2016;37(6):451‐462. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

