Long-term daily oral administration of intestinal permeation enhancers is safe and effective in mice
- PMID: 36684095
- PMCID: PMC9842030
- DOI: 10.1002/btm2.10342
Long-term daily oral administration of intestinal permeation enhancers is safe and effective in mice
Abstract
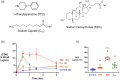
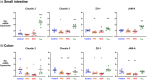
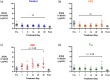
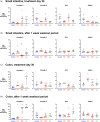
Although protein drugs are powerful biologic therapeutics, they cannot be delivered orally because their large size and hydrophilicity limit their absorption across the intestinal epithelium. One potential solution is the incorporation of permeation enhancers into oral protein formulations; however, few have advanced clinically due to toxicity concerns surrounding chronic use. To better understand these concerns, we conducted a 30-day longitudinal study of daily oral permeation enhancer use in mice and resultant effects on intestinal health. Specifically, we investigated three permeation enhancers: sodium caprate (C10), an industry standard, as well as 1-phenylpiperazine (PPZ) and sodium deoxycholate (SDC). Over 30 days of treatment, all mice gained weight, and none required removal from the study due to poor health. Furthermore, intestinal permeability did not increase following chronic use. We also quantified the gene expression of four tight junction proteins (claudin 2, claudin 3, ZO-1, and JAM-A). Significant differences in gene expression between untreated and permeation enhancer-treated mice were found, but these varied between treatment groups, with most differences resolving after a 1-week washout period. Immunofluorescence microscopy revealed no observable differences in protein localization or villus architecture between treated and untreated mice. Overall, PPZ and SDC performed comparably to C10, one of the most clinically advanced enhancers, and results suggest that the chronic use of some permeation enhancers may be therapeutically viable from a safety standpoint.
Keywords: intestinal epithelium; oral drug delivery; permeation enhancer.
© 2022 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


Similar articles
-
Intestinal permeation enhancers enable oral delivery of macromolecules up to 70 kDa in size.Eur J Pharm Biopharm. 2022 Jan;170:70-76. doi: 10.1016/j.ejpb.2021.11.010. Epub 2021 Dec 5. Eur J Pharm Biopharm. 2022. PMID: 34879228
-
A head-to-head Caco-2 assay comparison of the mechanisms of action of the intestinal permeation enhancers: SNAC and sodium caprate (C10).Eur J Pharm Biopharm. 2020 Jul;152:95-107. doi: 10.1016/j.ejpb.2020.04.023. Epub 2020 May 6. Eur J Pharm Biopharm. 2020. PMID: 32387703
-
Intestinal Permeation Enhancers for Oral Delivery of Macromolecules: A Comparison between Salcaprozate Sodium (SNAC) and Sodium Caprate (C10).Pharmaceutics. 2019 Feb 13;11(2):78. doi: 10.3390/pharmaceutics11020078. Pharmaceutics. 2019. PMID: 30781867 Free PMC article. Review.
-
Evaluation in pig of an intestinal administration device for oral peptide delivery.J Control Release. 2023 Jan;353:792-801. doi: 10.1016/j.jconrel.2022.12.011. Epub 2022 Dec 16. J Control Release. 2023. PMID: 36493948
-
Gastrointestinal Permeation Enhancers for the Development of Oral Peptide Pharmaceuticals.Pharmaceuticals (Basel). 2022 Dec 19;15(12):1585. doi: 10.3390/ph15121585. Pharmaceuticals (Basel). 2022. PMID: 36559036 Free PMC article. Review.
Cited by
-
Maternal milk cell components are uptaken by infant liver macrophages via extracellular vesicle mediated transport.FASEB J. 2025 Jan 31;39(2):e70340. doi: 10.1096/fj.202402365R. FASEB J. 2025. PMID: 39835705 Free PMC article.
-
Oral delivery of nanomedicine for genetic kidney disease.PNAS Nexus. 2024 May 10;3(5):pgae187. doi: 10.1093/pnasnexus/pgae187. eCollection 2024 May. PNAS Nexus. 2024. PMID: 38807632 Free PMC article.
-
Recent Progress in the Oral Delivery of Therapeutic Peptides and Proteins: Overview of Pharmaceutical Strategies to Overcome Absorption Hurdles.Adv Pharm Bull. 2024 Mar;14(1):11-33. doi: 10.34172/apb.2024.009. Epub 2023 Aug 26. Adv Pharm Bull. 2024. PMID: 38585454 Free PMC article. Review.
-
Evaluating the oral delivery of GalNAc-conjugated siRNAs in rodents and non-human primates.Nucleic Acids Res. 2024 Jun 10;52(10):5423-5437. doi: 10.1093/nar/gkae350. Nucleic Acids Res. 2024. PMID: 38742636 Free PMC article.
-
Storming the gate: New approaches for targeting the dynamic tight junction for improved drug delivery.Adv Drug Deliv Rev. 2023 Aug;199:114905. doi: 10.1016/j.addr.2023.114905. Epub 2023 Jun 3. Adv Drug Deliv Rev. 2023. PMID: 37271282 Free PMC article. Review.
References
-
- Benameur H. Capsule technology ‐ enteric capsule drug delivery technology ‐ achieving protection without coating. Drug Dev Deliv. 2015;15:1‐9.
-
- Wright JM, Jones GB. Developing the subcutaneous drug delivery route. Med Res Arch. 2017;5:1‐12.
LinkOut - more resources
Full Text Sources

