RNA therapeutics in the clinic
- PMID: 36684099
- PMCID: PMC9842029
- DOI: 10.1002/btm2.10374
RNA therapeutics in the clinic
Abstract
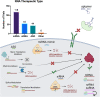
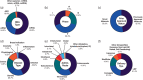
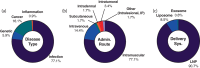
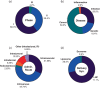
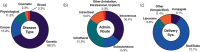
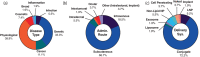
Ribonucleic acid (RNA) therapeutics are being actively researched as a therapeutic modality in preclinical and clinical studies. They have become one of the most ubiquitously known and discussed therapeutics in recent years in part due to the ongoing coronavirus pandemic. Since the first approval in 1998, research on RNA therapeutics has progressed to discovering new therapeutic targets and delivery strategies to enhance their safety and efficacy. Here, we provide an overview of the current clinically relevant RNA therapeutics, mechanistic basis of their function, and strategies to improve their clinical use. We discuss the 17 approved RNA therapeutics and perform an in-depth analysis of the 222 ongoing clinical trials, with an emphasis on their respective mechanisms and disease areas. We also provide perspectives on the challenges for clinical translation of RNA therapeutics and suggest potential strategies to address these challenges.
Keywords: RNA therapeutic; antisense oligonucleotide; aptamer; clinical trial; gene therapy; lipid nanoparticle; mRNA; mRNA vaccine; siRNA.
© 2022 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Figures
References
-
- US Department of Health and Human Services F and DA . Vaccines and Related Biological Products Advisory Committee December 17, 2020 meeting presentation: FDA review of efficacy and safety of Moderna COVID‐19 vaccine EUA.No Title; 2020. https://www.fda.gov/media/144585/download%0D
-
- US Department of Health and Human Services F and DA . Vaccines and Related Biological Products Advisory Committee December 10, 2020 meeting presentation: FDA review of efficacy and safety of Pfizer‐BioNTech COVID‐19 vaccine EUA; 2020.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

