Understudied factors in drug-coated balloon design and evaluation: A biophysical perspective
- PMID: 36684110
- PMCID: PMC9842065
- DOI: 10.1002/btm2.10370
Understudied factors in drug-coated balloon design and evaluation: A biophysical perspective
Abstract
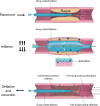
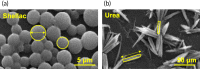
Drug-coated balloon (DCB) percutaneous interventional therapy allows for durable reopening of the narrowed lumen via physical tissue expansion and local anti-restenosis drug delivery, providing an alternative to traditional uncoated balloons or a permanent indwelling implant such as a conventional metallic drug-eluting stent. While DCB-based treatment of peripheral arterial disease (PAD) has been incorporated into clinical guidelines, DCB use has been recently curtailed due to reports that showed evidence of increased mortality risk in patients treated with paclitaxel (PTX)-coated balloons. Given the United States Food and Drug Administration's 2019 consequent warning regarding PTX-eluting DCBs and the subsequent marked reduction in clinical DCB use, there is now a critical need to better understand the compositional and mechanical factors underlying DCB efficacy and safety. Most work to date on DCB refinement has focused on designing both the enabling balloon catheter and alternate coatings composed of various drugs and excipients, followed by device evaluation in preclinical and clinical studies. We contend that improvement in DCB performance will require a better understanding of the biophysical factors operative during and following balloon deployment, and moreover that the elaboration and demonstrated control of these factors are needed to address current concerns with DCB use. This article provides a perspective on the biophysical interactions that govern DCB performance and offers new design strategies for the development of next-generation DCB devices.
Keywords: arterial disease; drug‐coated balloon; stenosis; vascular drug delivery.
© 2022 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Figures




Similar articles
-
Intrinsic coating morphology modulates acute drug transfer in drug-coated balloon therapy.Sci Rep. 2019 May 2;9(1):6839. doi: 10.1038/s41598-019-43095-9. Sci Rep. 2019. PMID: 31048704 Free PMC article.
-
The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty.JACC Cardiovasc Interv. 2014 Jan;7(1):10-9. doi: 10.1016/j.jcin.2013.05.022. JACC Cardiovasc Interv. 2014. PMID: 24456716 Clinical Trial.
-
Critical appraisal of paclitaxel balloon angioplasty for femoral-popliteal arterial disease.Vasc Health Risk Manag. 2016 Aug 29;12:341-56. doi: 10.2147/VHRM.S81122. eCollection 2016. Vasc Health Risk Manag. 2016. PMID: 27621646 Free PMC article. Review.
-
Systematic review and updated meta-analysis of the use of drug-coated balloon angioplasty versus plain old balloon angioplasty for femoropopliteal arterial disease.J Vasc Surg. 2019 Sep;70(3):981-995.e10. doi: 10.1016/j.jvs.2019.01.080. Epub 2019 May 21. J Vasc Surg. 2019. PMID: 31126769
-
Design and rationale of a prospective, randomized, non-inferiority trial to determine the safety and efficacy of the Biolimus A9™ drug coated balloon for the treatment of in-stent restenosis: First-in-man trial (REFORM).Cardiovasc Revasc Med. 2023 Nov;56:75-81. doi: 10.1016/j.carrev.2023.06.004. Epub 2023 Jun 6. Cardiovasc Revasc Med. 2023. PMID: 37328392 Clinical Trial.
Cited by
-
Cardiovascular Tissue Engineering Models for Atherosclerosis Treatment Development.Bioengineering (Basel). 2023 Nov 29;10(12):1373. doi: 10.3390/bioengineering10121373. Bioengineering (Basel). 2023. PMID: 38135964 Free PMC article. Review.
-
Preliminary Evaluation of 3D-Printed Alginate/Gelatin Scaffolds for Protein Fast Release as Suitable Devices for Personalized Medicine.Biomedicines. 2025 Jun 2;13(6):1365. doi: 10.3390/biomedicines13061365. Biomedicines. 2025. PMID: 40564084 Free PMC article.
-
Hydrophilic Coating Microstructure Mediates Acute Drug Transfer in Drug-Coated Balloon Therapy.ACS Appl Bio Mater. 2024 May 20;7(5):3041-3049. doi: 10.1021/acsabm.4c00080. Epub 2024 Apr 25. ACS Appl Bio Mater. 2024. PMID: 38661721 Free PMC article.
-
Drug-Coated Balloons in Percutaneous Coronary Intervention: Beyond Evidence.Cureus. 2024 Nov 4;16(11):e72990. doi: 10.7759/cureus.72990. eCollection 2024 Nov. Cureus. 2024. PMID: 39634989 Free PMC article. Review.
-
Choosing the right treatment for the right lesion, Part II: a narrative review of drug-coated balloon angioplasty and its evolving role in dialysis access maintenance.Cardiovasc Diagn Ther. 2023 Feb 28;13(1):233-259. doi: 10.21037/cdt-22-497. Epub 2023 Jan 9. Cardiovasc Diagn Ther. 2023. PMID: 36864970 Free PMC article. Review.
References
-
- Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509‐1526. - PubMed
-
- Schillinger M, Haumer M, Schlerka G, et al. Restenosis after percutaneous transluminal angioplasty in the femoropopliteal segment: the role of inflammation. J Endovasc Ther. 2001;8(5):477‐483. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

