Photothermally responsive theranostic nanocomposites for near-infrared light triggered drug release and enhanced synergism of photothermo-chemotherapy for gastric cancer
- PMID: 36684111
- PMCID: PMC9842049
- DOI: 10.1002/btm2.10368
Photothermally responsive theranostic nanocomposites for near-infrared light triggered drug release and enhanced synergism of photothermo-chemotherapy for gastric cancer
Abstract
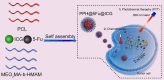
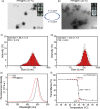
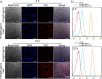
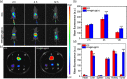
Near-infrared (NIR) photothermal therapy plays a critical role in the cancer treatment and diagnosis as a promising carcinoma treatment modalities nowadays. However, development of clinical application has been greatly limited due to the inefficient drug release and low tumor accumulation. Herein, we designed a NIR-light triggered indocyanine green (ICG)-based PCL core/P(MEO2MA-b-HMAM) shell nanocomposites (PPH@ICG) and evaluated their therapeutic effects in vitro and in vivo. The anticancer drug 5-fluorouracil (5Fu) and the photothermal agent ICG were loaded into a thermo-sensitive micelle (PPH@5Fu@ICG) by self-assembly. The nanoparticles formed were characterized using transmission electron microscopy, dynamic light scattering, and fluorescence spectra. The thermo-sensitive copolymer (PPH@5Fu@ICG) showed a great temperature-controlled drug release response with lower critical solution temperature. In vitro cellular uptake and TEM imaging proved that PPH@5Fu@ICG nanoparticles can home into the lysosomal compartments under NIR. Moreover, in gastric tumor-bearing nude mice, PPH@5Fu@ICG + NIR group exhibited excellent improvement in antitumor efficacy based on the NIR-triggered thermo-chemotherapy synergy, both in vitro and in vivo. In summary, the proposed strategy of synergistic photo-hyperthermia chemotherapy effectively reduced the 5Fu dose, toxic or side effect, which could serve as a secure and efficient approach for cancer theranostics.
Keywords: chemotherapy; gastric cancer; nanocarriers; photothermal therapy; photothermally responsive.
© 2022 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

