Predictive value of systemic immune-inflammation index for pathological complete response in patients receiving neoadjuvant immunochemotherapy for locally advanced esophageal cancer
- PMID: 36684142
- PMCID: PMC9845901
- DOI: 10.3389/fsurg.2022.1091601
Predictive value of systemic immune-inflammation index for pathological complete response in patients receiving neoadjuvant immunochemotherapy for locally advanced esophageal cancer
Abstract
Objectives: Neoadjuvant immunochemotherapy (nICT) has been confirmed with promising pathological complete response (pCR) among locally advanced esophageal squamous cell carcinoma (ESCC). However, there were still no reliable and accurate predictors to predict the treatment response. This study aimed to explore the predictive value of inflammatory and nutritional parameters.
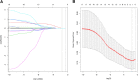
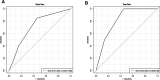
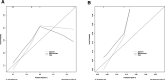
Methods: Patients with ESCC who underwent radical surgery after nICT between January 2020 and April 2022 were included in the study. First, the least absolute shrinkage and selection operator regression (LASSO) logistic regression analysis was used to screen independent inflammatory and nutritional parameters. Secondly, univariate and multivariate logistic regression were used to screen and predict independent risk factors for pCR. Thirdly, a nomogram was constructed based on the independent predictive factors, and 30% of the included population was randomly selected as the validation cohort. We used the receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA) curve to evaluate the nomogram model.
Results: A total of 97 ESCC patients were screened for analysis, with 20 patients with pCR (20.32%). Only the systemic immune-inflammation index (SII) was screened after LASSO-logistic regression when λ was 0.06. The cut-off value of SII was 921.80 with an area under curve (AUC) value of 0.62. We defined SII > 921.80 as high SII and SII ≦ 921.80 as low SII. Further, the univariate and multivariate analysis further determined SII(OR = 3.94, 95%CI:1.26-12.42, P = 0.02) and clinical stage(OR = 0.35, 95%CI:0.12-0.98, P = 0.05) were independent predictive factors of pCR. One novel nomogram was established with an AUC value of 0.72 in the training cohort and 0.82 in the validation cohort. The Brier score of the calibration curve was 0.13. The calibration curve showed good agreement between the predicted results and the actual results in both the training cohort and the validation cohort. Compared with the clinical stage, the DCA confirmed a better clinical value of the nomogram model in both the training cohort and the validation cohort.
Conclusions: High pretreatment SII and early clinical stage were independently associated with pCR among ESCC receiving nICT. We further established and validated one novel nomogram model to effectively predict pCR among ESCC after nICT.
Keywords: esophageal squamous cell carcinoma; neoadjuvant immunochemotherapy; nomogram model; pathological complete response; systemic immune-inflammatory index.
© 2023 Han, Weng, Zhang and Hong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20(11):1506–17. 10.1016/S1470-2045(19)30626-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

