Hormone Receptor and HER2 Status Switch in Non-pCR Breast Cancer Specimens after Neoadjuvant Therapy
- PMID: 36684405
- PMCID: PMC9851067
- DOI: 10.1159/000524698
Hormone Receptor and HER2 Status Switch in Non-pCR Breast Cancer Specimens after Neoadjuvant Therapy
Abstract
Introduction: This project aimed to identify the frequency of a switch of hormone receptor (HR) and/or HER2 status after neoadjuvant chemotherapy (NAC) for early breast cancer.
Methods: Tumor samples from patients without pathological complete response (non-pCR) were evaluated. Pathological complete response (pCR) was defined as no invasive tumor in breast and lymph nodes (ypT0/is ypN0). HR and HER2 status determined before NAC was compared with the corresponding receptor status determined in the surgical specimen after NAC.
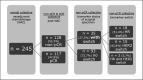
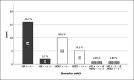
Results: 245 consecutive patients with primary invasive breast cancer, treated with NAC with/without targeted therapy between January 1, 2016 and December 31, 2019, at the LMU Breast Center, Munich, Germany, were identified. In 128 patients (52%), surgery revealed non-pCR after completed NAC. In 35 cases (27%), a switch of either HR and/or HER2 status between the initial biopsy and the surgical specimen was detected. Twenty cases had a switch in HR status, while 15 cases had a switch in HER2 status.
Conclusion: In a substantial number (27%) of non-pCR cases, a switch in biomarker status after completed neoadjuvant treatment was detected. These results are consistent with prior evidence. Yet, routine reevaluation of HR and HER2 status is not recommended in guidelines so far. Future research needs to address the impact of HR and HER2 status switch on therapy adaptation and on subsequent patient outcome. Particularly, in view of the recent therapy advances, it will be critical to evaluate whether individualization of treatment concepts based on the biology of the non-pCR specimens is preferable to the initial therapy concept based on the pathology at primary diagnosis.
Keywords: Biomarker switch; Breast cancer; Neoadjuvant chemotherapy; Pathological complete response.
Copyright © 2022 by S. Karger AG, Basel.
Conflict of interest statement
M. Dimpfl has no conflicts of interest to declare. D. Mayr has no conflicts of interest to declare. E. Schmoeckel received lecture fees from Roche. T. Degenhardt received honoraria for lectures and/or advisory board from Pfizer and Roche. Travel support from Tesaro, Daiichi, Sankyo. T. K. Eggersmann received honoraria for lectures and/or consulting from Roche, Novartis, Pfizer, and Aristo Pharma. N. Harbeck reports honoraria for lectures and/or consulting from Amgen, AstraZeneca, Daiichi Sankyo, Exact Sciences, Lilly, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Sandoz, and Seagen. R. Wuerstlein served as advisor consultant, speaker, and travel grant for Agendia, Amgen, Aristo, Astra Zeneca, Boeringer Ingelheim, Carl Zeiss, Celgene, Clinsol, Daiichi-Sankyo, Eisai, Exact Sciences, Genomic Health, Gilead, Glaxo Smith Kline, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Nanostring, Novartis, Odonate, Paxman, Palleos, Pfizer, Pierre Fabre, PumaBiotechnology, Riemser, Roche, Sandoz/Hexal, Seattle Genetics/Seagen, Tesaro Bio, Veracyte, and Viatris.
Figures
Similar articles
-
De-escalated neoadjuvant pertuzumab plus trastuzumab therapy with or without weekly paclitaxel in HER2-positive, hormone receptor-negative, early breast cancer (WSG-ADAPT-HER2+/HR-): survival outcomes from a multicentre, open-label, randomised, phase 2 trial.Lancet Oncol. 2022 May;23(5):625-635. doi: 10.1016/S1470-2045(22)00159-0. Epub 2022 Apr 8. Lancet Oncol. 2022. PMID: 35405088 Clinical Trial.
-
Factors Affecting Pathological Complete Response After Neoadjuvant Chemotherapy in Operable Primary Breast Cancer.J Coll Physicians Surg Pak. 2020 Apr;30(4):389-393. doi: 10.29271/jcpsp.2020.04.389. J Coll Physicians Surg Pak. 2020. PMID: 32513358
-
Neoadjuvant Chemotherapy and Pathologic Complete Response in HR+/HER2- Breast Cancer: Impact of Tumor Ki67 and ER Status.Chemotherapy. 2024;69(3):141-149. doi: 10.1159/000537874. Epub 2024 Feb 16. Chemotherapy. 2024. PMID: 38368871
-
Neoadjuvant Therapy for HER2-positive Breast Cancer.Rev Recent Clin Trials. 2017;12(2):81-92. doi: 10.2174/1574887112666170202165049. Rev Recent Clin Trials. 2017. PMID: 28164759 Review.
-
Ki67 and lymphocytes in the pretherapeutic core biopsy of primary invasive breast cancer: positive markers of therapy response prediction and superior survival.Horm Mol Biol Clin Investig. 2017 Sep 22;32(2):/j/hmbci.2017.32.issue-2/hmbci-2017-0022/hmbci-2017-0022.xml. doi: 10.1515/hmbci-2017-0022. Horm Mol Biol Clin Investig. 2017. PMID: 28937963 Review.
References
-
- Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. 2019 Sep 23;5((1)):66. - PubMed
-
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008 Feb 10;26((5)):778–785. - PubMed
-
- Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013 Oct;24 Suppl 6:vi7–23. - PubMed
-
- Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol. 2017 Aug 1;28((8)):1700–1712. - PMC - PubMed
-
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014 Jul 12;384((9938)):164–172. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous